Topology-guided functional multiplicity of iron(III)-based metal–organic frameworks†
Erika
Virmani
a,
Ole
Beyer
 b,
Ulrich
Lüning
b,
Ulrich
Lüning
 b,
Uwe
Ruschewitz
b,
Uwe
Ruschewitz
 c and
Stefan
Wuttke
c and
Stefan
Wuttke
 *a
*a
aDepartment für Chemie und Center for NanoScience (CeNS), Ludwig-Maximilians-Universität München, Butenandtstraße 11, D-81377 München, Germany. E-mail: stefan.wuttke@cup.uni-muenchen.de
bChristian-Albrechts-Universität zu Kiel, Otto Diels-Institut für Organische Chemie, Otto-Hahn-Platz 4, D-24118 Kiel, Germany
cUniversität zu Köln, Institut für Anorganische Chemie, Greinstr. 6, D-50939 Köln, Germany
First published on 26th July 2017
Abstract
We report here the synthesis and characterization of a new series of mixed-linker iron(III)-based metal–organic frameworks (MOFs) consisting of dicarboxylate linkers (1,4-benzenedicarboxylic acid ≡ BDC or its amino functionalized derivative) and tricarboxylate linkers (4,4′,4′′-[1,3,5-triazine-2,4,6-triyl]tribenzoic acid ≡ TATB or its nitro functionalized derivative). The resulting mesoporous MOFs with MIL-143 topology are stable under ambient water conditions for 14 d regardless of the functionalization of the organic linkers. Powder X-ray diffraction results reveal high crystallinity of the materials. This structure type is very tolerant to variation in the functional groups (e.g. nitro and/or amino) along the BDC and/or TATB linkers, but is less tolerant to changes in the size of the linkers themselves. It was attempted to replace linear BDC by biphenyl-4,4′-dicarboxylic acid (BPDC) and trigonal TATB by 2,4-bis(4′-carboxy-biphenyl-4-yl)-6-(4′-carboxy-2-methoxy-biphenyl-4-yl)-1,3,5-triazine (TAPB). Of the three additional structures made possible by combinations of these linkers (BDC/TAPB, BPDC/TATB, BPDC/TAPB), only one (BDC/TAPB) yields a crystalline product which, like the BDC/TATB crystal, exhibits MIL-143 topology. However, this material is not very stable and collapses upon guest removal. Our results suggest that the incorporation of diverse functional groups on linkers with different geometries in this new iron(III)-based MOF series offers a simple method of precisely tuning the chemical environment within the pores. More importantly, our work expands the scope of mixed-linker MOFs to include a subset of multivariate MOFs characterized by different functionalities in each type of linker.
Introduction
Metal–organic framework (MOF) crystal engineering is based on the study of periodically assembled metal ions or metal clusters (inorganic building units or IBUs, also known as secondary building units or SBUs) and organic linkers (organic building units or OBUs). This concept allows control over the structure of MOFs with respect to their topology. This ability to control structure, composition, porosity, and properties on the molecular level promises potential applications in gas storage1–3 and gas separation,4–6 catalysis,7,8 chemical sensing,9,10 and drug release.11–13 However, all of these applications are dependent upon the presence of chemical functionality either at the IBU and/or OBU.Multivariate MOFs (MTV-MOFs) are constructed from a parent MOF structural backbone along which different functionalities are distributed.14–18 The disordered spatial arrangement of the functionalities allows the MTV-MOFs to create unique synergistic effects.14 The random arrangement of the functionalities makes it difficult to draw conclusions about the relationship between structure, spatial arrangement and performance of the MTV-MOFs. Determining the location of randomly dispersed functionalities within MTV-MOFs is a huge scientific challenge that has recently been addressed with a combination of multidimensional solid-state NMR and molecular simulations.19 This challenge, however, can be circumvented by deliberately placing different functional linkers at specific, defined positions in the MOF scaffold.
By using the so-called mixed linker approach, two or more non-functionalized OBUs can be precisely incorporated into a MOF scaffold with predetermined structural topologies. Three approaches for synthesizing mixed linker MOFs are reported so far. The first approach creates a MOF composed of linkers with different binding sites (mostly carboxylic groups and electron donating nitrogen atoms), but the same linker topology.20–22 The second approach creates a MOF that is built from different linker topologies but the same binding sites.3,23,24 The third approach is a combination of the previous two by using linkers with different binding sites and different topologies.25
Interestingly, up to now only one functional group has been incorporated in MOFs using the mixed linker approach and the functionalization occurs so far only at linear dicarboxylic acid linkers. Therefore, the MOF is assembled by using one functionalized dicarboxylic acid linker and one non-functionalized linker. In this way, nitro, methyl, amino, fluoro, chloro, bromo, hydroxy, diacetoxy, sulfone, sulfonic acidband dioxole groups16,23,26–35 or different alkyloxy or alkyl groups and even alkyl ether groups of the type –O–(CH2)n–O–CH336–38 have been successfully incorporated into MOF scaffolds. The amino-functionalization of 1,4-benzenedicarboxylic acid has already been addressed through post-synthetic modification with acetic anhydride and derivatives with different alkyl chain length.39,40 Recently the Zhou group41 reported a mixed linker MOF where methyl and amino functional groups had been incorporated at two linker backbones. This MOF is constructed of three linear dicarboxylic acid linkers of varied length (2-amino-1,4-dicarboxylate, 2,2′-dimethylbiphenyl-4,4′-dicarboxylate and 2′,5′-dimethoxyterphenyl), and can only be obtained through a multiple step synthesis protocol.
Herein we report the synthesis of new iron(III)-based mixed linker MOFs exhibiting OBUs with different geometries (i.e. linear and triangular) and functionalities (nitro and/or amino). Using different combinations of functionalized and non-functionalized 1,4-benzenedicarboxylic acid (BDC) and 4,4′,4′′-(1,3,5-triazine-2,4,6-triyl)tribenzoic acid (TATB) linkers, it was possible to synthesize MOFs exhibiting the mesoporous MIL-143 topology. After the syntheses of the organic linkers and the corresponding iron(III)-based mixed linker MOFs, the materials were fully characterized in terms of their crystal structure, morphology, porosity, thermal and water stability. In addition, we describe the extension of either the linear BDC, by replacing it with biphenyl-4,4′-dicarboxylic acid (BPDC) or the trigonal TATB linker by replacing it with 2,4-bis(4′-carboxy-biphenyl-4-yl)-6-(4′-carboxy-2-methoxy-biphenyl-4-yl)-1,3,5-triazine (TAPB) which led to MOFs with reduced crystallinities and stabilities.
Experimental
Linker synthesis
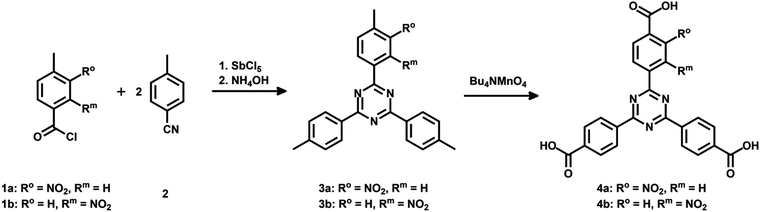
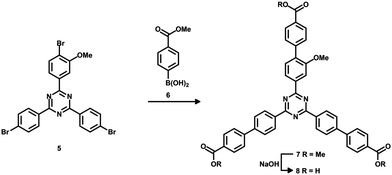
4,4′,4′′-(1,3,5-Triazine-2,4,6-triyl)tribenzoic acid (TATB) and two mono-substituted nitro-TATB linker versions were synthesized as described in the literature.42Fig. 1 illustrates the synthesis in which the trimerization of one equivalent of a nitro-substituted benzoyl chloride 1a or 1b with two equivalents of 4-methylbenzonitrile (2) leads to nitro-triazines 3a or 3b. By oxidizing the methyl groups with tetra-n-butylammonium permanganate, the respective nitro-TATB linkers 4a and 4b were obtained.Precursor 5 for the mono-substituted methoxy-TAPB linker 8 was obtained in a similar way. 4-Bromo-3-methoxy-benzoyl chloride was synthesized and used in the trimerization with two equivalents of 4-bromobenzonitrile to obtain tribromo-triazine 543 (for the synthetic route see ESI† Fig. S1). Triple Suzuki coupling with 4-(methoxycarbonyl)-phenylboronic acid (6) and alkaline hydrolysis of the methyl ester groups in 7 led to the desired methoxy-TAPB linker 8 (Fig. 2). In this paper, the synthesis was performed on a multigram scale with modifications in the last two steps (for further information see ESI†).
MOF synthesis
The Fe-TATB-BDC MOFs were synthesized in 5 mL Schott Duran culture tubes with a PBT cap equipped with a Teflon seal. FeCl3·6H2O (0.222 mmol) was dissolved in 1 mL DMF via ultrasonication. 4,4′,4′′-(1,3,5-Triazine-2,4,6-triyl)tribenzoic acid (TATB, 0.110 mmol) and 1,4-benzenedicarboxylic acid (BDC, 0.220 mmol), or their functionalized derivatives, were added and dissolved by ultrasonication. The reaction mixture was heated within 1 h to 150 °C, held at this temperature for 20 h and was afterwards cooled to room temperature within 1 h. The resulting powders were collected by centrifugation (5 min, 7830 rpm), washed three times with DMF and dried under reduced pressure at room temperature. The samples were afterwards extracted with methanol in a Soxhlet device for 72 h, dried again under reduced pressure and stored under nitrogen atmosphere at room temperature. For further synthetic details and the synthesis of the other iron(III)-based mixed linker MOF structures (Fe-TATB-BPDC, Fe-TAPB-BDC, Fe-TAPB-BPDC and Fe-BTB-BDC), see ESI.†Characterization
Powder X-ray diffraction (PXRD) data were recorded on a STOE Stadi MP diffractometer in transmission mode. The Cu Kα1 radiation source (λ = 1.54060 Å) was operating at 40 kV and 40 mA and the scattered X-rays were recorded on a DECTRIS MYTHEN 1 K solid state detector. High-resolution synchrotron powder diffraction data were recorded at the Swiss Norwegian BeamLine (SNBL BM01B) at the European Synchrotron at Grenoble/France (ESRF).44 The wavelength was calibrated with a Si standard NIST 640c to 0.504477 Å. The diffractometer is equipped with five counting channels, delivering five complete patterns collected simultaneously with a small 1.1° offset in 2θ. A Si(111) analyzer crystal was mounted in front of each NaI scintillator/photomultiplier detector. Data were collected at room temperature (Fe-TATB-BDC-a, -b, -c, -e) and 100 K (Fe-TATB-BDC-a, -e) between 0° and 20°/25° in 2θ with steps of 0.002° and 100 ms integration time per data point (approx. 20 min for one scan). This scanning procedure was repeated several times and the resulting data from all detectors and measurements were averaged into one pattern (Table S8 and Fig. S32, ESI†) with local software. Quantachrome AUTOSORB-1 or AUTOSOR-IQ instruments were used for measuring nitrogen sorption isotherms. The samples were degassed prior to the measurement at 120 °C for 12 h in vacuum. The calculation of the BET surface area was carried out in the p/p0 range of 0.005 to 0.01. A quenched solid state functional theory (QSDFT) was employed for calculating the pore size distribution. Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) measurements were determined in parallel using a Netzsch Jupiter ST 449 C instrument equipped with a Netsch TASC 414/4 controller. Scanning electron microscopy (SEM) images were recorded with a FEI Helios G33 UC. For further characterization of the materials see ESI.†Results and discussion
Different functionalized Fe-TATB-BDC MOFs
In this work, five different functionalized iron(III)-based MOF structures exhibiting two different linker geometries were synthesized. Fig. 3A illustrates the different functionalized linkers and the five combinations tested. Either 1,4-benzenedicarboxylic acid (BDC; red) or its amino-functionalized version (brown) were used as the linear linkers, and 4,4′,4′′-(1,3,5-triazine-2,4,6-triyl)tribenzoic acid (TATB; turquois) or its nitro-functionalized version (violet for meta position and blue for ortho position) were used as the trigonal linker.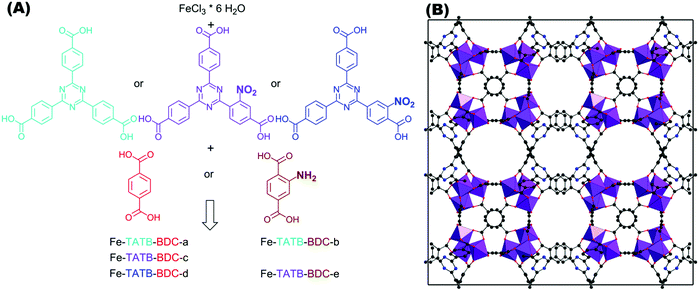 | ||
| Fig. 3 (A) Schematic representation of triazine-based trigonal linker TATB (turquois) and its mono-substituted nitro-functionalized version, in meta (violet) or ortho (blue) position to the connecting carboxylic acid group. The reactions of iron chloride hexahydrate with one TATB linker in combination with one of the two linear BDC linkers used (1,4-benzenedicarboxylic acid, illustrated in red and its amino-functionalized version illustrated in brown) result in different possible Fe-TATB-BDC MOF frameworks. Five different combinations were tested, resulting in the Fe-TATB-BDC structures indicated. (B) Presentation of the MIL-143 framework of Fe-TATB-BDC-a along [001] with FeO6 octahedra shown in purple, carbon as grey spheres, oxygen as red spheres, and nitrogen as blue spheres. Hydrogen atoms have been omitted for clarity. Note: the positional parameters have not been refined, but were taken from the literature.45 The cubic lattice constant was taken from our Le Bail fits and three carbon atoms of the benzene ring of the BTB linker were replaced by nitrogen atoms to obtain the TATB linker. | ||
While the BDC linkers are commercially available, the TATB ones had to be synthesized. The symmetrical functionalization of triazine-base materials can be easily achieved by a trimerization of nitriles. A modified synthetic route was chosen for the introduction of only one nitro group per linker molecule (Fig. 1). The nitro group was placed at different positions to investigate the effect on the resulting MOF structure and its properties.
The key to success in the synthesis of the five new iron(III)-based mixed linker compounds was the adaption of the synthesis protocol reported by Chevreau et al.45 In this report, they were able to synthesize MOFs with the MIL-142 and MIL-143 topologies for the first time. MOFs with the MIL-143 topology can be regarded as mesoporous zeolytic solids based on the β-christobalite topology, and the related MIL-142 topology is best described as a microporous interpenetrated structure similar to the ReO3 topology. The MIL-142 and MIL-143 structures are built up by the same linkers (BDC and BTB and their respective derivatives), but with different linker ratios (BDC to BTB is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 for MIL-142, while for MIL-143 the ratio is 3
4 for MIL-142, while for MIL-143 the ratio is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).45 Our goal was to synthesize MOFs exhibiting only the mesoporous MIL-143 topology (pore diameters: 2.0 nm and 2.4 nm; window sizes: 1.6 nm and 1.8 nm) as the synthesis of robust mesoporous MOFs is still a great challenge.
2).45 Our goal was to synthesize MOFs exhibiting only the mesoporous MIL-143 topology (pore diameters: 2.0 nm and 2.4 nm; window sizes: 1.6 nm and 1.8 nm) as the synthesis of robust mesoporous MOFs is still a great challenge.
For the successful synthesis, FeCl3·6H2O, trigonal TATB and linear BDC linkers were dissolved in DMF and the reaction mixture was heated at 150 °C for 20 h. The reaction mixture was purified by centrifugation, washing with DMF, and Soxhlet-extraction with methanol, which has proven to be much more effective in removing guest molecules – such as DMF – than washing alone.46 We also synthesized MIL-142 (hereafter called Fe-BTB-BDC with BTB = 4,4′,4′′-benzene-1,3,5-triyl-tris(benzoic acid), see ESI† and Fig. 9) according to our new synthesis protocol, to compare the chemical properties with the new Fe-TATB-BDC structures. The key difference between the Fe-BTB-BDC and the Fe-TATB-BDC structures is the trigonal linker, as the core of the trigonal linker in Fe-BTB-BDC is based on a benzene ring and not a triazine ring, as for Fe-TATB-BDC. Therefore, we were curious whether the enhancement of planarity through the substitution of the central benzene ring in BTB with a triazine core in the TATB linker would influence the properties (e.g. crystallinity, porosity, thermal and water stability) of the resulting MOF scaffold.
The PXRD patterns (Fig. 4) of the Fe-TATB-BDC structures indicate that the products are highly crystalline materials. The similarities between the PXRD patterns allow us to conclude that all five MOF samples crystallize in the same MOF topology. Comparison with calculated PXRD patterns based on the reported crystallographic data45 clearly prove that the MIL-143 topology is formed in all Fe-TATB-BDC compounds. Chevreau et al.45 were able to achieve the MIL-142 and MIL-143 topologies for combinations of BTB and BDC linkers by changing the relative amounts of starting materials as well as synthesis conditions. As we only obtained the MIL-143 topology, we assume that the trigonal linker based on a triazine core favors the formation of the non-interpenetrating MIL-143 topology, while the trigonal linker based on a benzene core favors the MIL-142 structure (Fig. S26, ESI†).
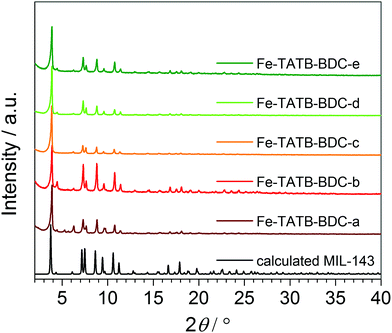 | ||
| Fig. 4 PXRD patterns of the five different functionalized Fe-TATB-BDC structures in comparison to the calculated pattern of the MIL-143 topology.45 | ||
The PXRD patterns shown in Fig. 4 were recorded on samples after Soxhlet-extraction, but exposure to air and moisture thereafter. To get more structural information high-resolution synchrotron powder diffraction data of selected compounds (Fe-TATB-a, -b, -c, and -e) were recorded at the European Synchrotron at Grenoble/France. Here the samples were activated (2 h at 5·10−2 mbar, 100 °C) and sealed in glass capillaries (∅ = 1.0 mm) to prevent the uptake of any water from the atmosphere.
A visual inspection and comparison with calculated PXRD patterns again confirmed the MIL-143 topology of these four compounds. But even with these high quality data no stable Rietveld refinement was achieved. Therefore, Le Bail fits in space group F23 using the GSAS software47 were conducted to obtain the cubic lattice parameters a for all compounds. The results of these fits (synchrotron data and laboratory X-ray data) are summarized in Table S8 and depicted in Fig. S32 and S33 (ESI†). The quality of the Le Bail fits of the synchrotron data is not as good as that of the STOE data. This is probably due to the fact that the synchrotron profiles cannot be accurately modelled with the functions implemented in GSAS. Nonetheless, these fits allow us to draw clear conclusions. All compounds Fe-TATB-BDC crystallize in the MIL-143 topology; the unit cell volume is not significantly influenced by changes in the functionalized linkers. Compounds Fe-TATB-BDC-b–d appear to be single-phase samples, whereas in the PXRD patterns of compounds Fe-TATB-BDC-a and -e small amounts of some MIL-142 impurity can be spotted. On the other hand, Fe-BTB-BDC gives a very good Le Bail fit in space group R3c with lattice parameters similar to those obtained in the original paper.45 No additional reflections that would suggest a MIL-143 impurity are observed. Another remarkable finding is the shrinkage of the unit cell volume upon activation (Table S8, ESI†). This shrinkage accounts for 1.5–2.7% of the original unit cell volume, indicating a remarkable degree of flexibility in the MIL-143 framework. Another noteworthy finding is the increase of the unit cell volume of the non-functionalized compound Fe-TATB-BDC-a upon cooling. Such a negative thermal expansion (NTE) of coordination polymers and MOFs is not rare and has already been reported before.48–50
In Fig. 5, the PXRD pattern collected for Fe-TATB-BDC-b (STOE Stadi P data) is compared with a pattern calculated from the lattice parameter of the Le Bail fit (Table S8, ESI†) and the positional parameters given for MIL-143 in the literature.45 The agreement is reasonable thus confirming the MIL-143 topology of this compound. Differences in the intensities especially at higher 2θ angles can be attributed to the fact that the theoretical pattern was only calculated with framework atoms, while the measurement was recorded on an as-synthesized sample with guest molecules in its pores. In Fig. 6, the recorded pattern of Fe-BTB-BDC is compared with a theoretical pattern of MIL-142 calculated as described above for MIL-143. Here, the similarity of both patterns is obvious thus confirming the MIL-142 topology of this compound.
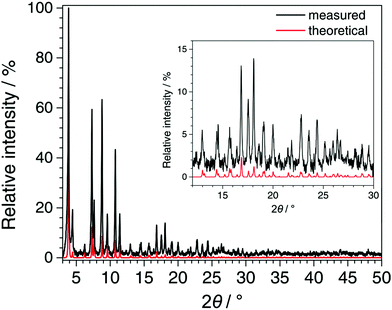 | ||
| Fig. 5 Comparison of the PXRD pattern recorded for Fe-TATB-BDC-b (STOE Stadi P, CuKα1 radiation, MYTHEN detector, background subtracted and zero shift corrected) with the pattern calculated from the refined lattice parameters of Fe-TATB-BDC-b (Le Bail fit) and the atomic coordinates of MIL-143 taken from the literature.45 The good agreement between the patterns confirms the MIL-143 topology of Fe-TATB-BDC-b. | ||
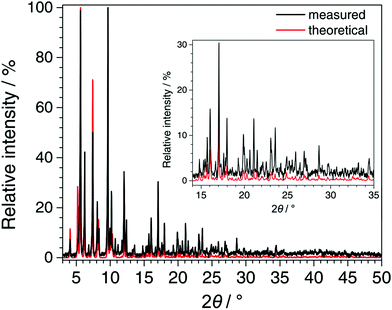 | ||
| Fig. 6 Comparison of the PXRD pattern recorded for Fe-BTB-BDC (STOE Stadi P, CuKα1 radiation, MYTHEN detector, background subtracted and zero shift corrected) with the pattern calculated from the refined lattice parameters of Fe-BTB-BDC (Le Bail fit) and the atomic coordinates of MIL-142 taken from the literature.45 The good agreement between the patterns confirms the MIL-142 topology of Fe-BTB-BDC. | ||
The SEM images of the Fe-TATB-BDC samples (Fig. S7, S10, S13, S16 and S19, ESI†) show intergrown aggregates with undefined particle shapes. However, on some images crystallites are visible and the sizes of the individual particles range from less than 100 nm to about 10 μm. Fe-BTB-BDC (Fig. S29, ESI†), in contrast, appears to be composed of plate-like hexagonal shaped crystals which is expected due to the rhombohedral space group R3c. The plates have similar sizes of approximately 400 nm thickness with edges lengths of 1.3–2.3 μm.
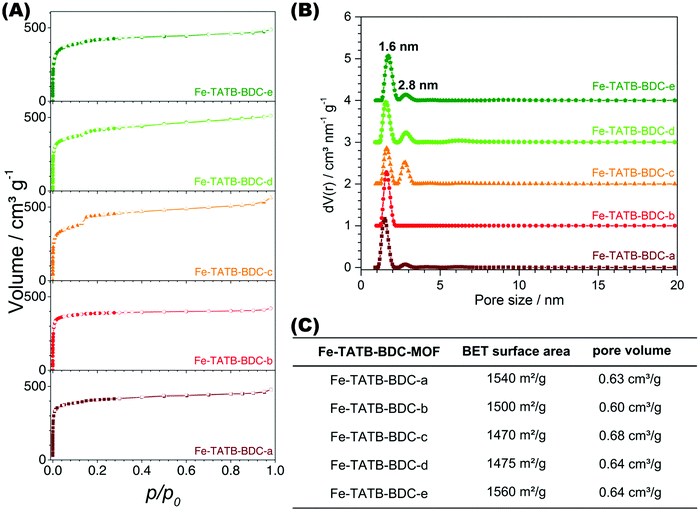
Fig. 7A presents the nitrogen isotherms of the Fe-TATB-BDC materials, which exhibit a Type IV(b) shape, except Fe-TATB-BDC-b which exhibits a Type I shape, according to IUPAC.51,52 The Type IV(b) isotherm, which had been recently added to the categorization of isotherms by IUPAC,52 is typical for mesoporous materials with pores smaller than 4 nm, while the Type I isotherm is typical for microporous materials. This Type IV(b) shape is in good agreement with the MIL-143 structure type, as the network is mesoporous and exhibits two different pore sizes of 2.0 nm and 2.8 nm. The presence of these two different pore widths is observed in the calculated pore size distribution, which shows two defined peaks (Fig. 7B). For all samples, except Fe-TATB-BDC-b, the pore size distribution indicates well-defined dimensions of about 1.6 nm and 2.8 nm, while Fe-TATB-BDC-b shows only one prominent pore with a diameter of 1.6 nm. One drastic difference in the pore size distributions of the MOFs is the relative amplitude of the second peak, which corresponds to the larger pores. This peak is significantly larger in the samples containing a nitro-functionalization at the trigonal TATB linker (Fe-TATB-BDC-c, -d, and -e). However, the irregularity in the presence and amplitude of a peak corresponding to the larger pore (2.8 nm) cannot be explained.
The BET surface areas of the five Fe-TATB-BDC materials fall within the same range: 1470–1560 m2 g−1 (Fig. 7C) regardless of which functional group is added or whether or not the linker is functionalized at all. Saturation of all isotherms was reached at p/p0 of 0.19, therefore this data point was used for the calculation of the pore volume. The pore volumes of the five Fe-TATB-BDC frameworks are also very similar at approximately 0.64 cm3 g−1. In comparison, for the Fe-BTB-BDC network, crystallizing in the MIL-142 topology, a BET surface area of 1585 m2 g−1 was observed (Fig. S27, ESI†). This is in a good agreement with the reported value of 1580 m2 g−1.45 The pore volume (0.62 cm3 g−1) was also calculated using a p/p0 value of 0.19 and is also in accordance with the value in the literature of 0.70 cm3 g−1.45 The shape of the isotherm of Fe-BTB-BDC (Fig. S27, ESI†) is a Type I, according to IUPAC,51,52 which is typical for microporous materials and consistent with the structure of MIL-142.45 The pore size distribution (Fig. S27, ESI†) is narrow with a maximum at 1.4 nm, slightly above the theoretical pore size (0.8 nm) of the MIL-142 topology.
Thermogravimetric analysis (TGA) was applied to determine the thermal stability and composition of the Fe-TATB-BDC frameworks (Fig. S6, S9, S12, S15 and S18, ESI†). The TGA curves indicate that the frameworks with unmodified linear BDC linkers (Fe-TATB-BDC-a, -c and -d; Fig. S6, S12 and S15, ESI†) are stable up to approximately 300 °C, while the frameworks containing an amino-functionalization (Fe-TATB-BDC-b and -e; Fig. S9 and S18, ESI†) are only stable up to 270 °C. These thermal stability value fits well with the stability of MIL-143 and the Fe-BTB-BDC framework (Fig. S28, ESI†), which are stable up to 315 °C.45 Hence, the triazine core of the trigonal linker has little influence on the resulting thermal stability of the framework. The main weight loss processes depicted in the TGA curves are more or less the same. The first main weight loss process is attributed to the removal of guest molecules. For Fe-TATB-BDC-a (Fig. S6, ESI†), the weight loss occurs until 315 °C and consists of two separated steps. These two steps of the curve indicate that the framework hosts two species of guest molecules, in this case water and DMF. In comparison, the TGAs of Fe-TATB-BDC-b (Fig. S9, ESI†) and -e (Fig. S18, ESI†) show a single weight loss step for the guest molecules up to 270 °C. Similarly, Fe-TATB-BDC-c (Fig. S12, ESI†) and -d (Fig. S15, ESI†) have a single weight loss step up to 300 °C. All of the MOFs also experience a second weight loss at higher temperatures, which can be attributed to the combustion of the organic linker. For Fe-TATB-a (Fig. S6, ESI†), -c (Fig. S12, ESI†) and -d (Fig. S15, ESI†) the weight loss process occurs in a single step that is finished at 410 °C, 400 °C and 430 °C, respectively. The TGA curves of Fe-TATB-b (Fig. S9, ESI†) and -e (Fig. S18, ESI†) indicate that bimodal linker decomposition occurs until approximately 480 °C. This can be attributed to the presence of amino groups at the linear BDC linker. The position of the nitro-functionalization seems to have a small effect on the thermal stability of the network, while the amino-functionalization has only an impact on the linker decomposition, but not on the thermal stability of the networks. In comparison, Fe-BTB-BDC (Fig. S28, ESI†) exhibits a defined two-step weight loss process in agreement with reported values of MIL-142.45 The first step (between 25 °C and 315 °C) can be attributed to the removal of guest molecules, while the second step (between 315 °C and 360 °C) can be attributed to the decomposition of the linkers. Assuming that at higher temperatures the residue only consisting of Fe2O3 and the molecular formula of MIL-143 to [Fe3O(Cl)(H2O)2(BDC)3/2(TATB)], the compositions of the Fe-TATB-BDC materials can be calculated (Tables S1–S5, ESI†). Comparison of these calculated compositions of the Fe-TATB-BDC MOFs with the data obtained from the TGA measurements (where an ideal network without guest molecules is denoted as guest-free) shows that the theoretical and experimental values agree and discrepancies fall within the range of accuracy of TGA measurements (1%). The same is true for Fe-BTB-BDC if the molecular formula is assumed to be [Fe3O(Cl)(H2O)2(BDC)(BTB)4/3] (Table S7, ESI†).
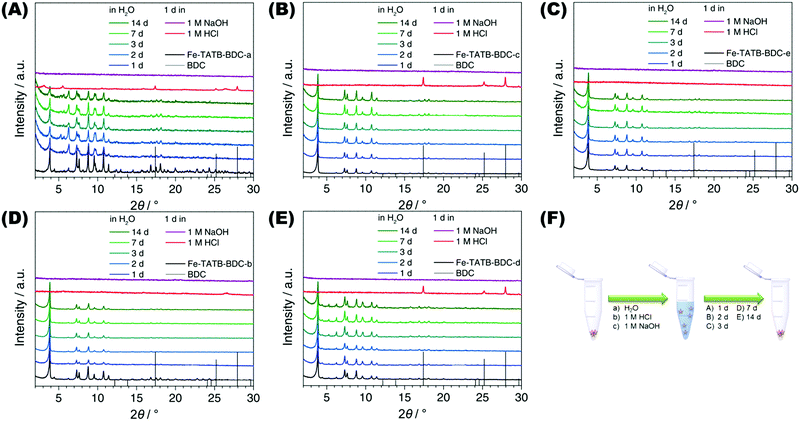
The stability of each MOF structure was tested in water and acidic or basic media. The samples were immersed in either Millipore water, 1 M hydrochloric acid, or 1 M sodium hydroxide. After different times of exposure, the MOF samples were then collected by centrifugation and dried at 100 °C before PXRD measurements were recorded. The PXRD data shown in Fig. 8 indicate that all five Fe-TATB-BDC frameworks are stable in water up to 14 d (Fig. 8, blue and green curves) but decompose under acidic (Fig. 8, red curves) and basic (Fig. 8, purple curves) conditions within one day. After the treatment under acidic conditions, BDC was detectable via PXRD for Fe-TATB-BDC-a, -c and -d (Fig. 8A, C and D, respectively). BDC was identified by comparing the reflections with theoretical patterns of BDC, shown as black line diagram in the PRXD plots (Fig. 8). This proves that the new synthesized iron(III)-based mix linker MOFs display similar chemical stability regardless of the functionalization of the organic linker. In most studies, iron(III)-based MOFs are members of the MIL (Matériaux de l'Institut Lavoisier) family and the framework stability in water increases from MIL-101(Fe),53,54 to MIL-88(Fe),55,56 further to MIL-53(Fe)57,58 and MIL-100(Fe).57–60 The newly synthesized iron(III)-based mixed linker MOFs exhibit a stability comparable to MIL-53(Fe) and MIL-100(Fe). MIL-53(Fe)57 is a flexible microporous material (with pores whose diameters range from 0.4 nm and 0.85 nm in the closed and opened form, respectively), while MIL-100(Fe)61 is a mesoporous material exhibiting pores of 2.5 nm and 2.9 nm. Compared to the newly synthesized iron(III)-based mixed linker materials, the surface and pores of MIL-100(Fe) are slightly larger. MIL-100(Fe) is constructed of benzene-1,3,5-tricarboxylic acid as the OBU and thus no functionalized MIL-100(Fe) network has been reported so far. In addition, the chemical stability of the Fe-TATB-BDC series is comparable to the Fe-BTB-BDC framework exhibiting a MIL-142 topology. The Fe-BTB-BDC framework (Fig. S30, ESI†) also decomposes under acidic and basic conditions, but is stable in water up to 14 d.
Elongation of the Fe-TATB-BDC network
In addition to the challenges associated with functionalizing the pore, developing a system to systematically expand the pore size of any given MOF structure is a daunting undertaking, but one that is crucial for making the MOF's surface accessible for large organic, inorganic, and biological molecules. Therefore, we tried to elongate the Fe-TATB-BDC framework by extending the length of both linkers (Fig. 9). First, we extended the linear linker BDC by replacing it with biphenyl-4,4′-dicarboxylic acid (BPDC) to achieve a Fe-TATB-BPDC framework. All our attempts led to a less crystalline material (Fig. S20, ESI†), but the PXRD pattern may indicate some first reflections of the Fe-TATB-BPDC framework. We then turned our attention to the elongation of the trigonal TATB linker by replacing it with 2,4-bis(4′-carboxy-biphenyl-4-yl)-6-(4′-carboxy-2-methoxy-biphenyl-4-yl)-1,3,5-triazine (TAPB). The respective MOF (Fe-TAPB-BDC) was obtained as a crystalline material (Fig. S21, ESI†) that also exhibits the MIL-143 topology. This was verified by a Le Bail fit in space group F23 leading to the cubic lattice parameter a = 50.910(8) Å. Attempts to obtain a structural model of Fe-TAPB-BDC failed due to the only modest data quality. It is remarkable that no interpenetration, that would stabilize the MOF structure, was observed. It seems that the triazine core of the TATB linker hinders interpenetration even when the networks are elongated, in contrast to the behavior of MOFs incorporating BTB linker.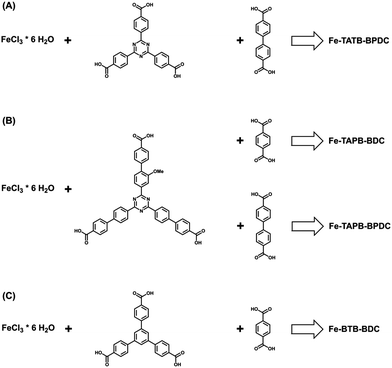 | ||
| Fig. 9 Scheme representing the additional tried combinations for the synthesis of iron(III)-based MOFs. (a) The elongation of the 1,4-benzenedicarboxylic acid was investigated, while (b) an elongation of the TATB linker was examined in combination with the 1,4-benzenedicarboxylic acid and its elongated version, biphenyl-4,4′-dicarboxylic acid. (c) Iron(III)-based MOF consisting of the trigonal linker BDC, this MOF is already known in the literature45 as MIL-142 or MIL-143 – isomeric structures with different linker contents – and was synthesized for comparison reasons. | ||
To our knowledge, this is the first time that an elongation of a MIL-143 structure type has been reported. Unfortunately, Soxhlet extraction with methanol led to an amorphous material (Fig. S21 (ESI†), dark cyan curve). Removing guest molecules in high vacuum at 120 °C or 50 °C for 12 h led to a collapse of the structure (Fig. S21 (ESI†), dark/light grey curves). Increasing the length of the organic linker is known to decrease the stability of a MOF structure.62 TGA was also used to determine the thermal stability of the framework, therefore the as-synthesized material was used. The curve shown in Fig. S22 (ESI†) indicates that multiple weight loss processes occurred. In comparison to the Fe-TATB-BDC networks, the first two weight loss steps (up to 315 °C) can be attributed to guest molecule removal. The third and fourth weight loss steps (up to 510 °C) can be attributed to the combustion of the linkers. The ideal composition of Fe-TAPB-BDC was calculated assuming the molecular formula to be [Fe3O(Cl)(H2O)2(BDC)3/2(TAPB)] (Table S6, ESI†). Based on these calculations the MOF framework seems to exhibit many defects (such as missing linkers), which could account for some of the observed reduced framework stability. SEM images (Fig. S23, ESI†) reveal intergrown octahedral shaped crystals with sizes of around 300 nm.
To complete our study, we also tried to elongate the network by extending both linkers at the same time. Instead of the desired Fe-TAPB-BPDC network, an amorphous material was obtained with only two broad reflections in the PXRD pattern (Fig. S24, ESI†).
Conclusion
In conclusion, five new mesoporous iron(III)-based mixed linker MOF materials exhibiting the non-interpenetrated MIL-143 topology were synthesized. Through the replacement of the central benzene ring of a BTB linker by a triazine core, the interpenetration of the network was avoided. As the MIL-143 topology is constructed from two different linker types, we were able to successfully synthesize MOFs that were functionalized by amino (linear BDC linker) or nitro groups (trigonal TATB linker). In the end, we were able to create a multivariate MOF with both amino and nitro groups by combining two differently functionalized linkers.All mesoporous iron(III)-based MOFs exhibit high thermal stability up to 300 °C. The water-stability is remarkable and comparable to the mesoporous MIL-100(Fe) structure. The frameworks are crystalline, display low defect concentrations, and their porosity is not affected by the functionalization of the linker molecules. We also demonstrated that the framework can be elongated by substituting the trigonal linker, but elongation of the linear linker leads to a less crystalline material. The resulting elongated framework of MIL-143, which has not been reported before, is unstable upon removal of guest molecules.
Apart from expanding the scope of mesoporous iron(III)-based MOFs, the strategy developed herein to synthesize multivariate mixed-linker MOFs, in which each linker can be functionalized separately, could lead to a better understanding of the relationship between functionality, arrangement, and performance of the MOF material.
Acknowledgements
We want to thank Tina Reuther (University of Munich) for nitrogen sorption and TGA/DSC measurements and Heidi Schwartz (University of Cologne) for preparing the samples for the investigations with synchrotron radiation as well as Dr Hermann Emerich for support at the SNBL beamline. Furthermore, we would like to thank Madeline Walden (Colorado College) for her valuable discussion and comments. SW is grateful for financial support from the Deutsche Forschungsgemeinschaft through DFG-project WU 622/4-1.References
- O. K. Farha, A. Ö. Yazaydin, I. Eryazici, C. D. Malliakas, B. G. Hauser, M. G. Kanatzidis, S. T. Nguyen, R. Q. Snurr and J. T. Hupp, Nat. Chem., 2010, 2, 944–948 CrossRef CAS PubMed.
- O. K. Farha, I. Eryazici, N. C. Jeong, B. G. Hauser, C. E. Wilmer, A. A. Sarjeant, R. Q. Snurr, S. T. Nguyen, A. Ö. Yazaydin and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 15016–15021 CrossRef CAS PubMed.
- H. Furukawa, N. Ko, Y. B. Go, N. Aratani, S. B. Choi, E. Choi, A. Ö. Yazaydin, R. Q. Snurr, M. O'Keeffe, J. Kim and O. M. Yaghi, Science, 2010, 329, 424–428 CrossRef CAS PubMed.
- J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2012, 112, 869–932 CrossRef CAS PubMed.
- L. J. Murray, M. Dincă and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294–1314 RSC.
- J. B. DeCoste and G. W. Peterson, Chem. Rev., 2014, 114, 5695–5727 CrossRef CAS PubMed.
- J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC.
- L. Ma, C. Abney and W. Lin, Chem. Soc. Rev., 2009, 38, 1248–1256 RSC.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- M. Allendorf, A. Bétard and R. A. Fischer, Metal–Organic Frameworks, Wiley-VCH Verlag GmbH & Co. KGaA, 2011, pp. 309–335 Search PubMed.
- J. An, S. J. Geib and N. L. Rosi, J. Am. Chem. Soc., 2009, 131, 8376–8377 CrossRef CAS PubMed.
- M. Lismont, L. Dreesen and S. Wuttke, Adv. Funct. Mater., 2017, 27, 1606314 CrossRef.
- S. Wuttke, M. Lismont, A. Escudero, B. Rungtaweevoranit and W. J. Parak, Biomaterials, 2017, 123, 172–183 CrossRef CAS PubMed.
- H. Deng, C. J. Doonan, H. Furukawa, R. B. Ferreira, J. Towne, C. B. Knobler, B. Wang and O. M. Yaghi, Science, 2010, 327, 846–850 CrossRef CAS PubMed.
- H. Furukawa, U. Müller and O. M. Yaghi, Angew. Chem., Int. Ed., 2015, 54, 3417–3430 CrossRef CAS PubMed.
- H. Chun, D. N. Dybtsev, H. Kim and K. Kim, Chem. – Eur. J., 2005, 11, 3521–3529 CrossRef CAS PubMed.
- W. Kleist, F. Jutz, M. Maciejewski and A. Baiker, Eur. J. Inorg. Chem., 2009, 3552–3561 CrossRef CAS.
- K. Koh, A. G. Wong-Foy and A. J. Matzger, Chem. Commun., 2009, 6162–6164 RSC.
- X. Kong, H. Deng, F. Yan, J. Kim, J. A. Swisher, B. Smit, O. M. Yaghi and J. A. Reimer, Science, 2013, 341, 882–885 CrossRef CAS PubMed.
- D. Senthil Raja, C.-C. Pan, C.-W. Chen, Y.-H. Kang, J.-J. Chen and C.-H. Lin, Microporous Mesoporous Mater., 2016, 231, 186–191 CrossRef CAS.
- J. Tao, M.-L. Tong and X.-M. Chen, J. Chem. Soc., Dalton Trans., 2000, 3669–3674 RSC.
- K. Seki, Chem. Commun., 2001, 1496–1497 RSC.
- C. J. Doonan, W. Morris, H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 9492–9493 CrossRef CAS PubMed.
- S. Yuan, J.-S. Qin, L. Zou, Y.-P. Chen, X. Wang, Q. Zhang and H.-C. Zhou, J. Am. Chem. Soc., 2016, 138, 6636–6642 CrossRef CAS PubMed.
- T.-T. Zhou, Z.-H. Xuan, D.-S. Zhang, Z. Chang, Y.-H. Zhang and X.-H. Bu, CrystEngComm, 2015, 17, 5884–5888 RSC.
- P. V. Dau, L. R. Polanco and S. M. Cohen, Dalton Trans., 2013, 42, 4013–4018 RSC.
- K. Uemura, F. Onishi, Y. Yamasaki and H. Kita, J. Solid State Chem., 2009, 182, 2852–2857 CrossRef CAS.
- T. Fukushima, S. Horike, Y. Inubushi, K. Nakagawa, Y. Kubota, M. Takata and S. Kitagawa, Angew. Chem., Int. Ed., 2010, 49, 4820–4824 CrossRef CAS PubMed.
- V. Safarifard and A. Morsali, CrystEngComm, 2014, 16, 8660–8663 RSC.
- A. Schoedel, W. Boyette, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, J. Am. Chem. Soc., 2013, 135, 14016–14019 CrossRef CAS PubMed.
- T. Yamada, S. Iwakiri, T. Hara, K. Kanaizuka, M. Kurmoo and H. Kitagawa, Cryst. Growth Des., 2011, 11, 1798–1806 CAS.
- T. Yamada and H. Kitagawa, J. Am. Chem. Soc., 2009, 131, 6312–6313 CrossRef CAS PubMed.
- K. Uemura, Y. Yamasaki, F. Onishi, H. Kita and M. Ebihara, Inorg. Chem., 2010, 49, 10133–10143 CrossRef CAS PubMed.
- S. Henke and R. A. Fischer, J. Am. Chem. Soc., 2011, 133, 2064–2067 CrossRef CAS PubMed.
- S. Horike, R. Matsuda, D. Tanaka, S. Matsubara, M. Mizuno, K. Endo and S. Kitagawa, Angew. Chem., Int. Ed., 2006, 45, 7226–7230 CrossRef CAS PubMed.
- S. Henke, R. Schmid, J.-D. Grunwaldt and R. A. Fischer, Chem. – Eur. J., 2010, 16, 14296–14306 CrossRef CAS PubMed.
- S. Henke, A. Schneemann, S. Kapoor, R. Winter and R. A. Fischer, J. Mater. Chem., 2012, 22, 909–918 RSC.
- S. Henke, A. Schneemann, A. Wutscher and R. A. Fischer, J. Am. Chem. Soc., 2012, 134, 9464–9474 CrossRef CAS PubMed.
- Z. Wang, K. K. Tanabe and S. M. Cohen, Inorg. Chem., 2009, 48, 296–306 CrossRef CAS PubMed.
- Z. Wang and S. M. Cohen, J. Am. Chem. Soc., 2009, 131, 16675–16677 CrossRef CAS PubMed.
- S. Yuan, W. Lu, Y.-P. Chen, Q. Zhang, T.-F. Liu, D. Feng, X. Wang, J. Qin and H.-C. Zhou, J. Am. Chem. Soc., 2015, 137, 3177–3180 CrossRef CAS PubMed.
- E. Mühlbauer, A. Klinkebiel, O. Beyer, F. Auras, S. Wuttke, U. Lüning and T. Bein, Microporous Mesoporous Mater., 2015, 216, 51–55 CrossRef.
- A. Klinkebiel, O. Beyer, B. Malawko and U. Lüning, Beilstein J. Org. Chem., 2016, 12, 2267–2273 CrossRef CAS PubMed.
- W. van Beek, O. V. Safonova, G. Wiker and H. Emerich, Phase Transitions, 2011, 84, 726–732 CrossRef CAS.
- H. Chevreau, T. Devic, F. Salles, G. Maurin, N. Stock and C. Serre, Angew. Chem., Int. Ed., 2013, 52, 5056–5060 CrossRef CAS PubMed.
- J. Lippke, B. Brosent, T. von Zons, E. Virmani, S. Lilienthal, T. Preuße, M. Hülsmann, A. M. Schneider, S. Wuttke, P. Behrens and A. Godt, Inorg. Chem., 2017, 56, 748–761 CrossRef CAS PubMed.
- A. C. Larson and R. B. Von Dreele, Los Alamos Lab., Rep. No. LA-UR, 1987, 86, 748 Search PubMed.
- F. Hohn, I. Pantenburg and U. Ruschewitz, Chem. – Eur. J., 2002, 8, 4536–4541 CrossRef CAS.
- S. R. G. Balestra, R. Bueno-Perez, S. Hamad, D. Dubbeldam, A. R. Ruiz-Salvador and S. Calero, Chem. Mater., 2016, 28, 8296–8304 CrossRef CAS PubMed.
- S. S. Han and W. A. Goddard III, J. Phys. Chem. C, 2007, 111, 15185–15191 CAS.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquérol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS.
- S. Liu, L. Zhai, C. Li, Y. Li, X. Guo, Y. Zhao and C. Wu, ACS Appl. Mater. Interfaces, 2014, 6, 5404–5412 CAS.
- P. Küsgens, M. Rose, I. Senkovska, H. Fröde, A. Henschel, S. Siegle and S. Kaskel, Microporous Mesoporous Mater., 2009, 120, 325–330 CrossRef.
- C. Serre, S. Surblé, C. Mellot-Draznieks, Y. Filinchuk and G. Férey, Dalton Trans., 2008, 5462–5464 RSC.
- P. Horcajada, F. Salles, S. Wuttke, T. Devic, D. Heurtaux, G. Maurin, A. Vimont, M. Daturi, O. David, E. Magnier, N. Stock, Y. Filinchuk, D. Popov, C. Riekel, G. Férey and C. Serre, J. Am. Chem. Soc., 2011, 133, 17839–17847 CrossRef CAS PubMed.
- I. Bezverkhyy, G. Weber and J.-P. Bellat, Microporous Mesoporous Mater., 2016, 219, 117–124 CrossRef CAS.
- X. Lan, H. Zhang, P. Bai and X. Guo, Microporous Mesoporous Mater., 2016, 231, 40–46 CrossRef CAS.
- E. Soubeyrand-Lenoir, C. Vagner, J. W. Yoon, P. Bazin, F. Ragon, Y. K. Hwang, C. Serre, J.-S. Chang and P. L. Llewellyn, J. Am. Chem. Soc., 2012, 134, 10174–10181 CrossRef CAS PubMed.
- Y.-K. Seo, J. W. Yoon, J. S. Lee, U.-H. Lee, Y. K. Hwang, C.-H. Jun, P. Horcajada, C. Serre and J.-S. Chang, Microporous Mesoporous Mater., 2012, 157, 137–145 CrossRef CAS.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J.-S. Chang, Y. K. Hwang, V. Marsaud, P.-N. Bories, L. Cynober, S. Gil, G. Férey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172–178 CrossRef CAS PubMed.
- I. Senkovska and S. Kaskel, Chem. Commun., 2014, 50, 7089–7098 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, additional material characterization for the linkers and the MOFs. See DOI: 10.1039/c7qm00263g |
| This journal is © the Partner Organisations 2017 |