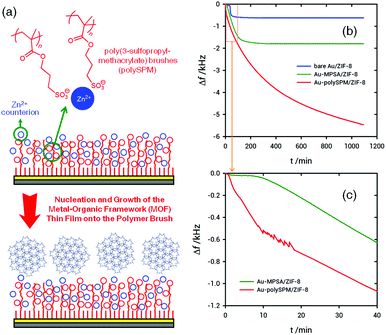
Metal–organic frameworks meet polymer brushes: enhanced crystalline film growth induced by macromolecular primers†
Matías
Rafti
 *a,
Juan A.
Allegretto
*a,
Juan A.
Allegretto
 ab,
Gustavo M.
Segovia
ab,
Gustavo M.
Segovia
 ab,
Jimena S.
Tuninetti
ab,
Jimena S.
Tuninetti
 a,
Juan M.
Giussi
a,
Elisa
Bindini
c and
Omar
Azzaroni
a,
Juan M.
Giussi
a,
Elisa
Bindini
c and
Omar
Azzaroni
 *a
*a
aInstituto de Investigaciones Fisicoquímicas Teóricas y Aplicadas (INIFTA), Departamento de Química, Facultad de Ciencias Exactas, Universidad Nacional de La Plata – CONICET, Diagonal 113 y 64, 1900 La Plata, Argentina
bUniversidad Nacional de San Martín (UNSAM), San Martín, Argentina
cLaboratoire Chimie de la Matière Condensée de Paris, UMR UPMC-CNRS 7574, Université Pierre et Marie Curie (Paris 6), 4 Place Jussieu, 75252 Paris, France
First published on 30th June 2017
Abstract
We used poly-(3-sulfopropylmethacrylate) brushes as macromolecular 3D primers to promote heterogeneous nucleation and growth of archetypal ZIF-8 MOF thin films. The enhancement can be understood in terms of a high preconcentration of Zn2+ ions in the polymer brush; this leads to a rapid increase of nucleation sites in the primer.
MOFs (metal–organic frameworks) represent an emergent class of porous materials constituted by infinite ordered arrays of metal-ion-based nodes or clusters, coordinated with multidentate organic linkers.1–3 Owing to their straightforward synthesis and many interesting features, such as unusually large surface areas, tuneable pore sizes and shapes and chemical versatility, MOFs find use in a growing number of applications such as sensors,4,5 catalysis,6–8 gas adsorption,9,10 and separations.11,12 The development of strategies for synthesizing MOF films has recently gained considerable attention13–16 due to their importance for the fabrication of separation membranes, optical devices and surface-mounted sensors.17–20 Different properties can be expected when considering polycrystalline films with thicknesses in the micrometre range as they can exhibit properties similar to bulk powders depending on the crystallite sizes. On the other hand, crystalline thin films (also known as SURMOFs) span over a few nm and can be regarded ideally as single-crystal domains with structures sometimes non-existing for their bulk counterparts.17,21,22 It was recently demonstrated that SURMOF growth can be highly dependent on the crystal orientation.23 Moreover, selective adsorption or “breathing” transitions can be observed for certain MOFs that do not present such behaviour when used as bulk powders.24,25
MOF films can be synthesized using a number of different procedures such as direct growth,4 layer-by-layer,26 solvothermal,27 colloidal28 or seeded growth.29 In all of these cases, the choice of an adequate chemical primer represents a crucial step towards achieving high crystallinity, good mechanical stability and enhanced growth extent.30 There are several examples of MOF films displaying a given preferential crystalline orientation obtained using this bottom-up assembly strategy, which ultimately relies on careful selection of monolayer primers exposing suitable chemical moieties.25,31–34
To date, the use of chemical primers to grow MOF thin films has been almost exclusively circumscribed to the use of self-assembled monolayers.35 The versatility of these molecular systems offers a simple means of modifying surfaces with predefined functional groups, which ultimately act as nucleation sites. However, due to the “monolayer” character of these systems, the number of functional groups – or nucleation sites – that can be allocated on a particular substrate is limited. In recent years, different authors started to explore the use of polymeric substrates as primers to grow MOF thin films. In a seminal work, Hatton and co-workers demonstrated that MIL-47 can be directly synthesized on polyacrylonitrile (PAN) substrates, providing thus evidence for the first time that MOFs can grow at the expense of an amorphous precursor phase.36 More recently, Caro and co-workers further extended these notions by using polydopamine layers as platforms to grow ZIF-8 thin films, showing that macromolecular surfaces can promote the heterogeneous nucleation of MOF crystals.37,38
Inspired by the above-described attempts directed to the creation of hybrid polymer–MOF materials,39 which would certainly help in broadening the range of possible applications by circumventing common limitations (e.g., organic phase immiscibility or sensitivity towards hydrolysis),40,41 we have explored the use of polymer brushes as macromolecular primers to promote the heterogeneous nucleation and growth of ZIF-8 thin films. Polymer brushes refer to assemblies of macromolecules that are tethered by one end to a surface or interface in which chemical groups all along the polymer backbone can be placed in pseudo-3D spatial arrangements. Polymer brushes provide a complementary perspective from which to consider the manipulation of the heterogeneous nucleation of MOF thin films as they expose a high density of moieties interacting with the precursors in the solution.42 Despite all the efforts made to optimize the heterogeneous nucleation of MOF thin films, little – or nothing – is known about the effect of exposing specific primers or functional groups in “polymeric” formats rather than in “monolayer” configurations. As such, this work aimed to address how the heterogeneous nucleation of MOF thin films is affected when the same functional group is introduced in the substrate as a “polymer brush” rather than as a self-assembled monolayer. Our proof-of-concept consists of employing densely grafted poly-(3-sulfopropyl-methacrylate) or poly-SPM brushes in order to explore the capabilities of this macromolecular primer to enhance the crystalline growth of ZIF-8 films.43
ZIF-8 (a member of the MOF subclass known as zeolitic imidazolate frameworks, with a sodalite (SOD) zeolite-type structure widely used as a prototype material in the literature)44,45 is a MOF constituted by tetrahedrally coordinated Zn2+ metal ions with bidentate 2-methylimidazolate (2-mIm) organic linkers. Aside from its reproducible straightforward synthesis and robustness, ZIF-8 was selected as a suitable candidate to explore the effect of multiple sulfonate moieties present on the brush, due to the already reported enhancement effect on nucleation caused by sulfonate-decorated surfaces.30,46,47 Our research demonstrates that in the presence of polymer brushes, the heterogeneous nucleation and growth of MOF thin films is promoted greatly; meanwhile, the resulting films still exhibit a smooth and homogeneous surface. As far as we know, this work constitutes the first systematic examination of “polymer brushes” as macromolecular primers to induce and enhance MOF thin film growth.
The preparation of poly-SPM brushes was carried out using aqueous, surface-initiated, atom transfer radical polymerization (SI-ATRP) of the SPM monomers following a previously reported procedure.43 The assembly procedure of ZIF-8 MOF on the poly-SPM modified surface is shown schematically in Fig. 1. Polymer brushes synthesized through overnight polymerization were characterized by FTIR, AFM and ellipsometry corroborating the presence of smooth polymer layers, with an average thickness of ≈250 nm (see ESI,† for characterization and synthesis details).
The SPM monomer was in the form of a potassium salt, and hence, the synthesized polymer brush is mainly coordinated with potassium ions as counter-ions. The large content of sulfonate groups in the polymer brush provides a great capacity for uptake and exchange of metal ions.48 This is one of the essential aspects of polymer brushes as primers for the nucleation of MOF thin films. Polyanionic polymer brushes can act as surface-confined nanoreservoirs for cations, such as Zn2+, that subsequently will play a decisive role as nucleation sites for the ZIF-8 film. In addition, the great advantage of polymer brushes is the enhanced accessibility between the functional groups and the solution given by the stretched conformation of the surface-grafted chains. In this way, species dissolved in a solution can interact more freely with the functional groups on the brush layer compared to the chemical moieties confined inside a compact polymeric substrate.
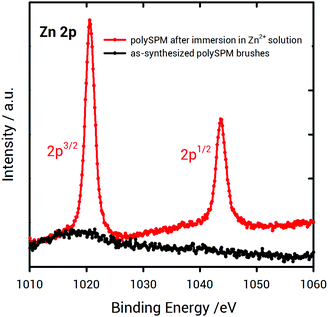
To demonstrate the ability of poly-SPM brushes to coordinate Zn2+ ions available from the solution, XPS experiments were conducted. XPS characterization confirmed the presence of Zn2+ in the brush layer after soaking the as-synthesized, K+-coordinated poly-SPM brushes for 20 seconds in a 50 mM Zn(NO3)2 methanolic solution (Fig. 2). This indicates that, even in the case of short immersion times, in the presence of Zn2+ ion-rich solutions, ion exchange occurs. As a result, K+ ions are removed from the macromolecular environment of the polymer brush and subsequently replaced by Zn2+ ions.
Once we had demonstrated that poly-SPM brushes can act as Zn2+ ion nanoreservoirs, we proceeded with the synthesis of ZIF-8 films using poly-SPM as a macromolecular primer. As stated above, the main aim of our work is to address how the growth of MOF thin films is affected when the heterogeneous nucleation occurs on a “macromolecular” or a “monomolecular” priming layer. To this end, we tracked the nucleation and growth of ZIF-8 films by performing in situ quartz crystal microbalance (QCM) experiments using Au-coated sensors modified with poly-SPM brushes. For the sake of comparison, similar experiments were performed using Au-coated sensors modified with a sodium 3-mercapto-1-propanesulfonate (MPSA) self-assembled monolayer. We have recently reported that sulfonate-bearing self-assembled monolayers are very efficient primers for the nucleation and growth of ZIF-8 films.30 In essence, the comparison of QCM traces describing ZIF-8 growth on poly-SPM brushes, and MPSA monolayers refers to the heterogeneous nucleation of the MOF layer at interfaces exposing sulfonate groups in “polymeric” and “monomolecular” configurations.
To evaluate influence of sulfonate moiety “interfacial configuration” on ZIF-8 film growth, in situ monitoring of growth kinetics using a quartz crystal microbalance (QCM) was carried out, as can be observed in Fig. 1. QCM experiments show time evolution of the frequency change (Δf) for films grown on QCM sensors modified with MPSA monolayers and poly-SPM brushes, respectively, after direct mixing of methanolic solutions of precursors at a stoichiometric metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) linker (1
linker (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) molar ratio (see ESI,† for further details).
2) molar ratio (see ESI,† for further details).
Remarkable differences can be observed, not only in terms of the mass/thickness of ZIF-8 films grown on both the sensor surfaces, but also regarding the early stages of film growth. QCM data indicate that the induction times, i.e., the time required to the start of film growth after mixing the precursor solutions, for surfaces modified with poly-SPM brushes and MPSA monolayers are 50 seconds and 9 minutes, respectively. In addition, it is observed that the long-time mass limit for ZIF-8 films grown on poly-SPM brushes is significantly higher than those grown on MPSA-modified surfaces. Microgravimetric experiments show striking differences between both the platforms after ≈150 min of film growth. The growth rate of ZIF-8 films for the MPSA-modified sensors is 370 ng cm−2 min−1 during the first 120 min, and then film growth stops. However, when using poly-SPM, the initial growth rate determined by QCM is 940 ng cm−2 min−1. Then, the growth process spans far beyond the first 120 min whereas the growth rate gradually changes from 370 to 25 ng cm−2 min−1 during a 16 hour period. Compared to MPSA 2D-primers, the still high value of growth rate even after initial stages have passed (370 ng cm−2 min−1) suggests that the influence of the poly-SPM 3D-primer spans beyond the obvious nucleation enhancement observed for early stages of film growth. As is well known, the induction period is influenced by supersaturation, i.e., the driving force necessary for the nucleation and growth of the crystalline phase. This induction time involves not only the time required for the formation of nuclei, but also the time needed for nuclei formed to attain detectable size.49 If we consider that poly-SPM brushes efficiently pre-concentrate Zn2+ ions, it is plausible to consider that the high concentration of nucleation sites hosted in the macromolecular layer will speed up the appearance of stable growing nuclei.50 Then, these nuclei will grow, spread and coalesce forming the MOF layer through a mechanism resembling multinucleation-multilayer growth.51
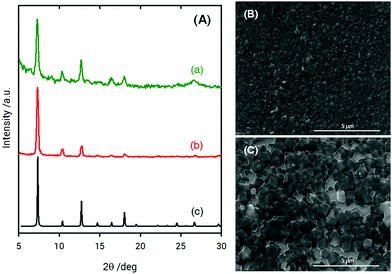
X-ray diffraction (XRD) measurements of ZIF-8 films grown on poly-SPM brushes were used to establish the crystallinity of the obtained material. Fig. 3 shows the XRD patterns collected for ZIF-8 thin films grown on poly-SPM- and MPSA-modified QCM substrates corresponding to the experiments presented in Fig. 1. The XRD patterns of the as-obtained films agree well with the expected patterns in terms of peak positions, thus confirming that ZIF-8 was present in both platforms, and providing strong evidence that the use of an amorphous polyelectrolyte brush as a priming layer has no detrimental effects on MOF crystallinity. Moreover, the noise-to-signal ratio is improved when using the poly-SPM primer. Further examination of SEM experiments showed noticeable morphological differences between ZIF-8 films when grown on poly-SPM- or MPSA-modified substrates. SEM imaging corresponding to the final state achieved in QCM experiments from Fig. 1(b) is presented in Fig. 3(B). It can be observed that when poly-SPM brushes were used as a primer, well-intergrown films were obtained (see the ellipsometry results in the ESI,† for additional evidence of smoothness achieved). The merged grains constituting the film have a relatively uniform, homogeneous size around 100–200 nm. On the contrary, a rough and poorly intergrown ZIF-8 film, rather than a continuous and smooth layer, was formed on the MPSA-functionalized substrates, as displayed in Fig. 3(C). This can be rationalized by assuming that the heterogeneous nucleation of ZIF-8 crystals on MPSA is relatively poor compared with poly-SPM surfaces. Once again, this observation can be rationalized if we consider that poly-SPM brushes act as an interfacial preconcentrator of Zn2+ species. The preconcentration of species that trigger the formation of nucleation sites shifts the system to a low supersaturation condition52 which, in turn, leads to a smoother film. Or, in other words, the preconcentration of Zn2+ ions within the polymer brush helps the system reach a critical interfacial supersaturation for the nucleation and growth of the MOF crystalline phase.53 It could be argued that polymer brushes would adopt partially collapsed configurations due to the moderate polar environment of the solvent used for synthesis, thus reducing the effective number of –SO3− moieties exposed to the solution. However, due to the roughness of the brush–solution interface, the comparison hereby presented with MPSA SAMs still allows for a good qualitative comparison between a 2D primer and an eminently 3D primer, such as the hypothetically partially collapsed poly-SPM brush. Once the ZIF-8 layer on the polyelectrolyte brush is grown, we explored the subsequent growth of MOF multilayers by sequential crystallization processes. This strategy directed towards growth of thick MOF films was referred to before as “sequential one-pot” (SOP)18,54 synthesis, and consists in simply dipping the substrate in a freshly prepared solution of precursors during a preset growth time (for the experiments shown in Fig. 4, 10 min). Homogeneous and heterogeneous nucleation processes occur simultaneously, and the (maximum) film thickness can be controlled by the number of SOP cycles performed. The multi-layer growth of ZIF-8 films on poly-SPM brushes was monitored by spectroscopic ellipsometry (see ESI,† file for further details). Fig. 4 shows successful “nanolayer-by-nanolayer” (NbN) film deposition55–58 with linear growth (≈70 nm per cycle), thus demonstrating that each crystallization cycle resulted in deposition of nearly the same amount of material. Compared with the traditional methods for preparing MOF thin films, our “nanolayer-by-nanolayer” strategy implemented on poly-SPM brushes achieved highly crystalline, thick films at high deposition rates in a rather controllable manner (e.g., it was previously reported that ≈30 min where needed to achieve similar thickness increase, with poorer control over surface rugosity).59
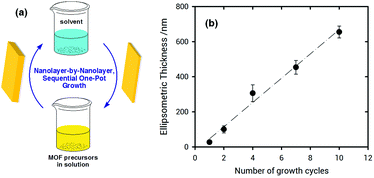 | ||
| Fig. 4 (a) Schematic illustration of the multilayer fabrication method. (b) Nanolayer-by-nanolayer (NbN), sequential one-pot growth of a ZIF-8 film onto a poly-SPM-modified Si/SiO2 substrate, as observed by spectroscopic ellipsometry (see ESI,† for details on modelling of the layers present). | ||
Conclusions
Controlling the growth, crystallinity and morphology of Metal Organic Framework (MOF) films is a challenging and highly desirable task due to the many applications discovered in the last few years. We hereby proposed for the first time the use of a 3D polymeric primer, with pre-defined multiple chemical moieties exposed. Taking advantage of the already demonstrated affinity of Zn2+ ions for the multiple sulfonate exposed moieties, we explored the possibility of an enhancement on heterogeneous nucleation. XPS characterization supports our working hypothesis; i.e., poly-SPM brushes can act as efficient interfacial preconcentrators of Zn2+ species. The preconcentration triggers the formation of nucleation sites, thus shifting the system to a low supersaturation condition. Using this approach, we were able to synthesize highly inter-grown, controlled thickness, smooth ZIF-8 films. A notorious enhancement in growth kinetics, and extent (3-fold increase versus 2D primer MPSA SAMs) was obtained as evidenced by QCM in situ measurements. We foresee that this novel strategy with high versatility could be applied with little modification for creating tailored polymeric 3D primers exposing virtually any desired moiety suitable for the growth of a wide range of MOFs.Notes and references
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341(80), 1230444 CrossRef PubMed.
- J. R. Long and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1213–1214 RSC.
- J. Jiang and O. M. Yaghi, Chem. Rev., 2015, 115, 6966–6997 CrossRef CAS PubMed.
- G. Lu and J. T. Hupp, J. Am. Chem. Soc., 2010, 132, 7832–7833 CrossRef CAS PubMed.
- Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815–5840 RSC.
- D. Farrusseng, S. Aguado and C. Pinel, Angew. Chem., Int. Ed., 2009, 48, 7502–7513 CrossRef CAS PubMed.
- J. Chen, R. Liu, Y. Guo, L. Chen and H. Gao, ACS Catal., 2015, 5, 722–733 CrossRef CAS.
- J. Gascon, A. Corma, F. Kapteijn and F. X. Llabrés i Xamena, ACS Catal., 2014, 4, 361–378 CrossRef CAS.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC.
- J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2012, 112, 869–932 CrossRef CAS PubMed.
- K. A. Cychosz and A. J. Matzger, Langmuir, 2010, 26, 17198–17202 CrossRef CAS PubMed.
- B. Van de Voorde, B. Bueken, J. Denayer and D. De Vos, Chem. Soc. Rev., 2014, 43, 5766–5788 RSC.
- S. Furukawa, J. Reboul, S. Diring, K. Sumida and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5700–5734 RSC.
- K. Otsubo, T. Haraguchi, O. Sakata, A. Fujiwara and H. Kitagawa, J. Am. Chem. Soc., 2012, 134, 9605–9608 CrossRef CAS PubMed.
- G. Xu, T. Yamada, K. Otsubo, S. Sakaida and H. Kitagawa, J. Am. Chem. Soc., 2012, 134, 16524–16527 CrossRef CAS PubMed.
- H. K. Arslan, O. Shekhah, J. Wohlgemuth, M. Franzreb, R. A. Fischer and C. Wöll, Adv. Funct. Mater., 2011, 21, 4228–4231 CrossRef CAS.
- A. Bétard and R. a. Fischer, Chem. Rev., 2012, 112, 1055–1083 CrossRef PubMed.
- O. Shekhah, J. Liu, R. a. Fischer and C. Wöll, Chem. Soc. Rev., 2011, 40, 1081–1106 RSC.
- A. Huang, H. Bux, F. Steinbach and J. Caro, Angew. Chem., Int. Ed., 2010, 49, 4958–4961 CrossRef CAS PubMed.
- A. L. Robinson, V. Stavila, T. R. Zeitler, M. I. White, S. M. Thornberg, J. A. Greathouse and M. D. Allendorf, Anal. Chem., 2012, 84, 7043–7051 CrossRef CAS PubMed.
- P. Falcaro, D. Buso, A. J. Hill and C. M. Doherty, Adv. Mater., 2012, 24, 3153–3168 CrossRef CAS PubMed.
- H. Gliemann and C. Wöll, Mater. Today, 2012, 15, 110–116 CrossRef CAS.
- O. Shekhah, H. Wang, D. Zacher, R. A. Fischer and C. Wöll, Angew. Chem., Int. Ed., 2009, 48, 5038–5041 CrossRef CAS PubMed.
- S. Sakaida, K. Otsubo, O. Sakata, C. Song, A. Fujiwara, M. Takata and H. Kitagawa, Nat. Chem., 2016, 8, 377–383 CrossRef CAS PubMed.
- B. Liu, M. Tu and R. a. Fischer, Angew. Chem., Int. Ed., 2013, 52, 3402–3405 CrossRef CAS PubMed.
- O. Shekhah and M. Eddaoudi, Chem. Commun., 2013, 49, 10079–10081 RSC.
- X. Zhang, Y. Liu, S. Li, L. Kong, H. Liu, Y. Li, W. Han, K. L. Yeung, W. Zhu, W. Yang and J. Qiu, Chem. Mater., 2014, 26, 1975–1981 CrossRef CAS.
- P. Horcajada, C. Serre, D. Grosso, C. Boissière, S. Perruchas, C. Sanchez and G. Férey, Adv. Mater., 2009, 21, 1931–1935 CrossRef CAS.
- Y. S. Li, H. Bux, A. Feldhoff, G. N. Li, W. S. Yang and J. Caro, Adv. Mater., 2010, 22, 3322–3326 CrossRef CAS PubMed.
- J. S. Tuninetti, M. Rafti and O. Azzaroni, RSC Adv., 2015, 5, 73958–73962 RSC.
- S. Hermes, F. Schröder, R. Chelmowski, C. Wöll and R. A. Fischer, J. Am. Chem. Soc., 2005, 127, 13744–13745 CrossRef CAS PubMed.
- S. Li, W. Shi, G. Lu, S. Li, S. C. J. Loo and F. Huo, Adv. Mater., 2012, 24, 5954–5958 CrossRef CAS PubMed.
- E. Biemmi, C. Scherb and T. Bein, J. Am. Chem. Soc., 2007, 129, 8054–8055 CrossRef CAS PubMed.
- P. Falcaro, K. Okada, T. Hara, K. Ikigaki, Y. Tokudome, A. W. Thornton, A. J. Hill, T. Williams, C. Doonan and M. Takahashi, Nat. Mater., 2017, 16, 342–348 CrossRef CAS PubMed.
- D. Zacher, O. Shekhah, C. Wöll and R. a. Fischer, Chem. Soc. Rev., 2009, 38, 1418–1429 RSC.
- A. Centrone, Y. Yang, S. Speakman, L. Bromberg, G. C. Rutledge and T. A. Hatton, J. Am. Chem. Soc., 2010, 132, 15687–15691 CrossRef CAS PubMed.
- A. Huang, Q. Liu, N. Wang, Y. Zhu and J. Caro, J. Am. Chem. Soc., 2014, 136, 14686–14689 CrossRef CAS PubMed.
- Q. Liu, N. Wang, J. Caro and A. Huang, J. Am. Chem. Soc., 2013, 135, 17679–17682 CrossRef CAS PubMed.
- M. Rafti, W. A. Marmisollé and O. Azzaroni, Adv. Mater. Interfaces, 2016, 3, 1600047 CrossRef.
- K. A. McDonald, J. I. Feldblyum, K. Koh, A. G. Wong-Foy and A. J. Matzger, Chem. Commun., 2015, 51, 11994–11996 RSC.
- W. Zhang, Y. Hu, J. Ge, H.-L. Jiang and S.-H. Yu, J. Am. Chem. Soc., 2014, 136, 16978–16981 CrossRef CAS PubMed.
- O. Azzaroni, S. E. Moya, A. a. Brown, Z. Zheng, E. Donath and W. T. S. Huck, Adv. Funct. Mater., 2006, 16, 1037–1042 CrossRef CAS.
- M. Ramstedt, N. Cheng, O. Azzaroni, D. Mossialos, H. J. Mathieu and W. T. S. Huck, Langmuir, 2007, 23, 3314–3321 CrossRef CAS PubMed.
- B. Wang, A. P. Côté, H. Furukawa, M. O'Keeffe and O. M. Yaghi, Nature, 2008, 453, 207–211 CrossRef CAS PubMed.
- K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- R. Zhang, S. Ji, N. Wang, L. Wang, G. Zhang and J.-R. Li, Angew. Chem., Int. Ed., 2014, 53, 9775–9779 CrossRef CAS PubMed.
- S. Bhattacharyya, S. H. Pang, M. R. Dutzer, R. P. Lively, K. S. Walton, D. S. Sholl and S. Nair, J. Phys. Chem. C, 2016, 120, 27221–27229 CAS.
- E. Choi, O. Azzaroni, N. Cheng, F. Zhou, T. Kelby and W. T. S. Huck, Langmuir, 2007, 23, 10389–10394 CrossRef CAS PubMed.
- J. W. Mullin, Crystallization, Butterworth-Heinemann, Oxford, 4th edn, 2001 Search PubMed.
- C. G. Pope, Microporous Mesoporous Mater., 1998, 21, 333–336 CrossRef CAS.
- M. Ohara and R. C. Reid, Prentice-Hall International Series in the Physical and Chemical Engineering Sciences: Modeling Crystal Growth Rates from Solution, Prentice-Hall, New Jersey, 1973 Search PubMed.
- D. Kashchiev and G. M. van Rosmalen, Cryst. Res. Technol., 2003, 38, 555–574 CrossRef CAS.
- P. Cubillas and M. W. Anderson, Zeolites and Catalysis: Synthesis, Reactions and Applications, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2010 Search PubMed.
- J. S. Tuninetti, M. Rafti, A. Andrieu-Brunsen and O. Azzaroni, Microporous Mesoporous Mater., 2016, 220, 253–257 CrossRef CAS.
- K. Ariga, Y. Yamauchi, G. Rydzek, Q. Ji, Y. Yonamine, K. C.-W. Wu and J. P. Hill, Chem. Lett., 2014, 43, 36–68 CrossRef CAS.
- K. Ariga, Q. Ji, W. Nakanishi, J. P. Hill and M. Aono, Mater. Horiz., 2015, 2, 406–413 RSC.
- K. Ariga, M. V. Lee, T. Mori, X.-Y. Yu and J. P. Hill, Adv. Colloid Interface Sci., 2010, 154, 20–29 CrossRef CAS PubMed.
- M. Aono, Y. Bando and K. Ariga, Adv. Mater., 2012, 24, 150–151 CrossRef CAS PubMed.
- C. Hou, Q. Xu, J. Peng, Z. Ji and X. Hu, ChemPhysChem, 2013, 14, 140–144 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7qm00235a |
| This journal is © the Partner Organisations 2017 |