Processing of Cr doped SrTiO3 nanoparticles into high surface area aerogels and thin films†
Felix
Rechberger
a,
Gabriele
Ilari
ab,
Christoph
Willa
a,
Elena
Tervoort
a and
Markus
Niederberger
 *a
*a
aLaboratory for Multifunctional Materials, Department of Materials, ETH Zurich, Vladimir-Prelog-Weg 5, CH-8093 Zurich, Switzerland. E-mail: markus.niederberger@mat.ethz.ch
bElectron Microscopy Center, EMPA, Swiss Federal Laboratories for Materials Science and Technology, 8600 Dubendorf, Switzerland
First published on 27th April 2017
Abstract
We present the nonaqueous sol–gel synthesis of crystalline SrTi1−xCrxO3 (x = 0, 0.3, 2, 5, 10%) nanoparticles and their processing into highly concentrated dispersions in ethanol by surface functionalization with 2-[2-(2-methoxyethoxy) ethoxy] acetic acid (MEEAA). These stable nanoparticle dispersions can then be assembled into 2- and 3-dimensional architectures such as films and aerogels. Homogeneous transparent films with a compact microstructure and a thickness of 140 nm are prepared from the dispersion by dip coating, while efficient destabilization and supercritical drying results in nanostructured bulk aerogels with a high surface area of up to 370 m2 g−1.
Introduction
SrTiO3 is a versatile perovskite material, which has gained significant attention due to its outstanding application potential in ferroelectrics,1 random access memories,2 photovoltaics,3 or photocatalysis.4 The low temperature synthesis of such alkaline-earth titanates and in particular SrTiO3 nanoparticles, has been pursued by many researchers using wet-chemical methods such as inverse micelle microemulsion,5,6 hydrothermal reactions7–10 and aqueous and nonaqueous sol–gel synthesis.6,11–14 Similar to TiO2, due to its large band gap of 3.2 eV, SrTiO3 is only able to generate electron–hole pairs through photoexcitation, when irradiated with high energy UV light. However, UV light only accounts for 5% of the solar spectrum. Therefore, significant efforts have been taken in order to modify the absorption edge of the material, thus rendering it photoactive also to visible light. Doping of a wide band gap semiconductor with a foreign element is an established method to achieve such absorption shifts to the visible range. For SrTiO3, reports of nitrogen,15,16 noble metal,17,18 transition metal19,20 and especially chromium doping4,21–29 are found to activate this material also to visible light. This development has led to a significant improvement in photocatalytic dye degradation,4,18–22 hydrogen production from water splitting,17,23–27,30 and recently also for CO2 reduction, currently a particularly hot topic.4,31,32 Incorporated in SrTiO3, chromium often exists in 3+ valence state, which is compensated by oxygen vacancies and acts as additional donor state in the valence band.33–35 In photocatalysis, apart from light absorption, a large contact area between photocatalyst and reactant is highly beneficial. Aerogels with their high surface area and low density offer both large interaction frequency and an interconnected pore system for high speed molecular transport and therefore aerogels are the ideal candidates for such applications.36The availability of highly concentrated and agglomeration-free dispersions is typically the first requirement for the successful processing of nanoparticles. Therefore, considerable efforts have been directed towards the development of efficient dispersion concepts under minimization of agglomeration and sedimentation. Unfortunately, basically for every system a unique, typically empirical combination of surface attached molecules and solvents is needed to produce stable dispersions unless the nanoparticles have similar surface properties. As BaTiO3 and SrTiO3 are both synthesized by the benzyl alcohol route, they should have very similar surface chemistry.37,38 This assumption motivated us to process Cr doped SrTiO3 nanoparticles in a similar way as we have previously showed in our group for BaTiO3.39–41 Functionalization with 2-[2-(2-methoxyethoxy) ethoxy] acetic acid (MEEAA) indeed allowed for dispersing the nanoparticles in high concentrations in ethanol (∼200 mg mL−1), remaining stable for months. Especially for the preparation of films as well as of 3-dimensional architectures like aerogels, highly concentrated dispersions greatly facilitate the production of such nanostructured materials.
For the production of thin films, the nanoparticle dispersions are processed by drop casting, dip- or spin coating while controlled destabilization of dispersions is an elegant method for the formation of nanoparticle based aerogels.42 In literature, reports on SrTiO3 based aerogels are very scarce. Klabunde et al. showed the synthesis of SrTiO3 aerogels from an ethanol–toluene mixture with a surface area of 159 m2 g−1.43 By doping with ruthenium and rhodium, the aerogels could be used for hydrogen generation from water splitting.44 In comparison to molecular routes, the assembly of preformed nanoparticles into macroscopic aerogels has gained a lot of attention in the recent years,42 as their compositional and thus also functional variety has greatly been expanded to metal chalcogenides,45 metal nitrides,46 noble metals47 and metal oxides.48–51 A major advantage of using particle based aerogels is that one can tune the desired properties from the materials point of view via a bottom-up approach, by developing building blocks that fulfill the requirements for a specific application, and these nanoparticle properties are then fully conserved in the macroscopic aerogel.
Here we present the synthesis and characterization of crystalline Cr doped SrTiO3 nanoparticles and the preparation of highly concentrated dispersions. Such dispersions are the prerequisite for their wet-chemical processing, which is exemplified based on two technologically particularly important geometries: thin films and macroscopic aerogels.
Results and discussion
The experimental details for the preparation of nanoparticles, their processing and characterization are given in the ESI.†The synthesis of Cr doped SrTiO3 nanoparticles followed a modified protocol by Niederberger et al.37 After heating of dissolved metallic strontium together with chromium(III) acetylacetonate and titanium(IV) isopropoxide in benzyl alcohol, highly crystalline Cr doped SrTiO3 nanoparticles were obtained, as shown by the powder X-ray diffraction (XRD) pattern of the as-synthesized powder (Fig. 1). The undoped sample showed no color, while Cr doping leads to a green coloration of the powder, indicating successful incorporation of Cr3+ into the SrTiO3 lattice. All samples were phase-pure and could clearly be assigned to cubic SrTiO3 (ICDD PDF No. 00-035-0734). The average crystal size, as estimated from the (110) peak broadening using the Scherrer equation, steadily decreased from 6.8 nm for undoped SrTiO3 to 4.5 nm for 10% Cr doping. The opposite trend, i.e. larger crystals for higher doping levels, was observed by Chang and Shen for SrTi1−xCrxO3 nanoparticles synthesized in ethylene glycol and citric acid.21 Even though the ionic radius of Cr3+ (0.62 Å) is very similar to the radius of the Ti4+ ion (0.61 Å),23 the increased chromium content suppresses grain growth during synthesis.22,29 Furthermore, Rietveld refinement of the unit cell parameters indicated an increase from a = 3.9174 Å for undoped SrTiO3 to 3.9309 Å for 10% Cr doping, as depicted in Table S1 (ESI†). The refined profiles are shown in Fig. S1 (ESI†). A detailed comparison of the (110) reflection of SrTi1−xCrxO3 in the range of 2θ = 30.5–34° (Fig. S2, ESI†) shows that the peak slightly shifts towards lower 2θ values while the intensity decreases with increasing doping level. This is in accordance with previous reports where Cr3+ cations substituted for Ti4+ result in a peak shift towards lower 2θ angles.23,25,26 Therefore, we believe that the Cr atoms were successfully incorporated into Ti sites of SrTiO3.
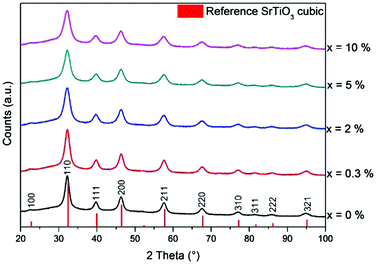 | ||
| Fig. 1 XRD diffractograms of as-synthesized SrTi1−xCrxO3 nanoparticles (vertical red bars show the reference pattern of cubic SrTiO3, ICDD PDF No. 00-035-0734). | ||
The organic species attached to the surface of the nanoparticles were analyzed by means of infrared (IR) spectroscopy and carbon elemental analysis. The IR spectra for different doping levels are very similar, indicating the same organic compounds to be present after synthesis. Therefore, only 10% Cr doped SrTiO3 spectra are shown as representative sample in Fig. S3 (ESI†). The strong IR vibrational bands in the range of 1300–1600 cm−1 for the as-synthesized SrTi0.9Cr0.1O3 nanoparticles can be assigned to aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of benzyl alcohol used as solvent during the synthesis.52 Pure unbound MEEAA shows a strong absorption peak at around 1100 cm−1, which can be attributed to the C–O–C bonds,41,53 and which is also found in the functionalized SrTiO3 particles. The absorption band at around 1751 cm−1 originates from C
C stretching of benzyl alcohol used as solvent during the synthesis.52 Pure unbound MEEAA shows a strong absorption peak at around 1100 cm−1, which can be attributed to the C–O–C bonds,41,53 and which is also found in the functionalized SrTiO3 particles. The absorption band at around 1751 cm−1 originates from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds of the carboxylic group.54 Peaks at 1300 cm−1, 1404 cm−1 and 1588 cm−1 can be ascribed to vibrations of the MEEAA COO− functional group after binding to the nanoparticles.41,54 We suggest, that MEEAA substitutes the surface-absorbed organics from the synthesis, as the C
O bonds of the carboxylic group.54 Peaks at 1300 cm−1, 1404 cm−1 and 1588 cm−1 can be ascribed to vibrations of the MEEAA COO− functional group after binding to the nanoparticles.41,54 We suggest, that MEEAA substitutes the surface-absorbed organics from the synthesis, as the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic absorption bands are not clearly visible anymore after functionalization. This is also in accordance to the report of Cheema and Garnweitner on MEEAA functionalized ZrO2 nanoparticles with a similar surface chemistry and from observations made for MEEAA functionalized BaTiO3.41,54 Carbon elemental analysis confirmed the increase in carbon content from 10% for as-synthesized particles to 17% after functionalization with MEEAA.
C aromatic absorption bands are not clearly visible anymore after functionalization. This is also in accordance to the report of Cheema and Garnweitner on MEEAA functionalized ZrO2 nanoparticles with a similar surface chemistry and from observations made for MEEAA functionalized BaTiO3.41,54 Carbon elemental analysis confirmed the increase in carbon content from 10% for as-synthesized particles to 17% after functionalization with MEEAA.
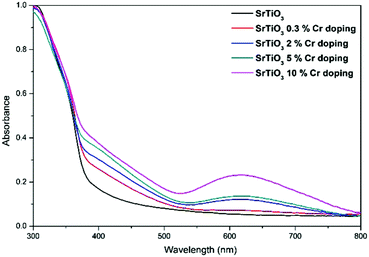
Fig. 2 shows the UV/vis absorption of the Cr doped SrTiO3 nanoparticles. It can be seen that the doped nanoparticles show a red-shifted onset of absorption in the visible-light region with an absorption edge beyond 500 nm, which is especially pronounced for the higher doping levels. Additionally, a broad peak at around 620 nm is observed for the doped samples, which could be ascribed to a d–d transition of 4A2–4T2 of the Cr3+.22–24,32,55 These results further indicate that Cr3+ has been successfully doped in SrTiO3 and results in a narrowing of the band gap.22–26,28,32
MEEAA functionalized SrTi1−xCrxO3 nanoparticles were easily dispersed in high concentration in ethanol. It is worth mentioning that the amount of Cr doping has an influence on the quality of the dispersion. The higher the dopant concentration, the clearer the dispersion gets. However, detailed reasons for this behavior are not yet understood. The resulting dispersions of doped SrTiO3 remain stable for months even without stirring or mechanical agitation, independent of the chromium content, while the undoped dispersion was slightly milky and stable for a few days only. A representative photograph is shown in Fig. 3 for a 10% Cr doped SrTiO3 dispersion in ethanol with a concentration of around 200 mg mL−1. The availability of such high quality dispersions is an important prerequisite for further processing of the nanoparticles into thin films and aerogel, which is the primary goal of this report.
In order to destabilize such dispersions in a controlled way to reach a gelled state,42 a modified protocol as previously published by our group for BaTiO3 aerogels was used.39 By adding water in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and heating the homogeneous mixture at 70 °C for 30 min, the previously clear and liquid dispersion turned slightly opaque and the entire sample volume is gelled. Solvent exchange to acetone allowed for supercritical drying under full preservation of the microstructure and the resulting macroscopic monolithic aerogels were cylindrically shaped and measured 1.5 × 1.5 cm as visualized in Fig. 4. The measured gravimetric density of the aerogels lies in the range of 0.22–0.1 g cm−3 for the undoped and doped samples. This corresponds to only 4.5–2.2% of the relative bulk density of SrTiO3, indicating an extremely porous structure. Carbon elemental analysis showed that the carbon content drops to below 5% as a result of the solvent exchange and supercritical drying. These values agree well with other particle based metal oxide aerogels.39,41,56 Compared to the as-prepared powders, no phase change was observed in the XRD pattern and no crystal growth was observed.
1 ratio and heating the homogeneous mixture at 70 °C for 30 min, the previously clear and liquid dispersion turned slightly opaque and the entire sample volume is gelled. Solvent exchange to acetone allowed for supercritical drying under full preservation of the microstructure and the resulting macroscopic monolithic aerogels were cylindrically shaped and measured 1.5 × 1.5 cm as visualized in Fig. 4. The measured gravimetric density of the aerogels lies in the range of 0.22–0.1 g cm−3 for the undoped and doped samples. This corresponds to only 4.5–2.2% of the relative bulk density of SrTiO3, indicating an extremely porous structure. Carbon elemental analysis showed that the carbon content drops to below 5% as a result of the solvent exchange and supercritical drying. These values agree well with other particle based metal oxide aerogels.39,41,56 Compared to the as-prepared powders, no phase change was observed in the XRD pattern and no crystal growth was observed.
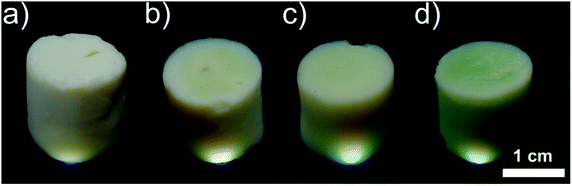 | ||
| Fig. 4 Photographs of monolithic SrTi1−xCrxO3 aerogels with x = (a) 0.3%, (b) 2%, (c) 5%, (d) 10%. The aerogel samples are illuminated from below to show the translucency. | ||
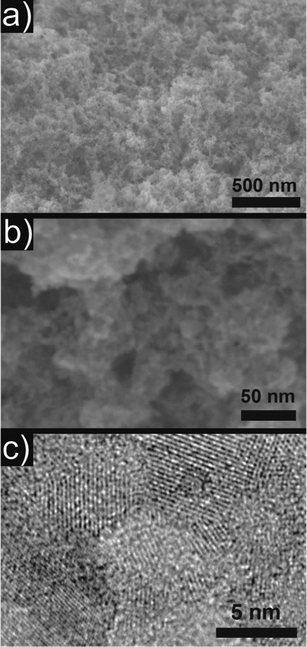
Scanning electron microscopy (SEM) investigations of the 10% Cr doped aerogel showed the highly porous microstructure with a random distribution of pores in the range of a few nm up to several hundred nm (Fig. 5a and b). It is important to note that no microstructural changes were observed for doped and undoped samples, therefore only a representative sample of 10% Cr doping is shown. This is a remarkable observation, as the dispersions of the doped nanoparticles seem to be of better quality, indicating the presence of smaller aggregates than the undoped sample. However, no differences were visible in the final aerogel. A transmission electron microscope (TEM) overview is given in Fig. S4 (ESI†), while high resolution transmission electron microscopy (HRTEM) in Fig. 5c confirmed the crystallite size of around 4.5 nm as obtained by XRD with random crystallographic orientation of the building blocks with respect to each other. In addition, the STEM-EDX analysis revealed that 3.35 ± 0.18 at% of the initial 10% Cr were incorporated in the SrTiO3 matrix, while for an initial concentration of 5%, a final incorporation of 1.21 ± 0.06% Cr occurs. The corresponding EDX spectra and mapping for 10% Cr is visualized in Fig. S5 and S6 (ESI†), indicating a homogeneous distribution of Cr within the sample. For lower doping levels (2% and 0.3% Cr), EDX is not sensitive enough to detect and quantify the remaining dopant. However, XRD unit cell refinement and UV/vis spectroscopy could clearly identify the incorporation of Cr in the SrTiO3 host even at low concentrations. Therefore, we are convinced that Cr is successfully incorporated into SrTiO3.
Based on diffraction, spectroscopy and imaging techniques, we propose an assembly mechanism similar to BaTiO3 aerogels, where water partially removes MEEAA from random crystal facets. This partial destabilization results in the formation of a three-dimensional network by attachment of nanoparticles at random facets, supported by the high concentration of the initial dispersion.39,42 Thermal agitation of the reaction mixture ensures a rapid and effective destabilization and gelation.
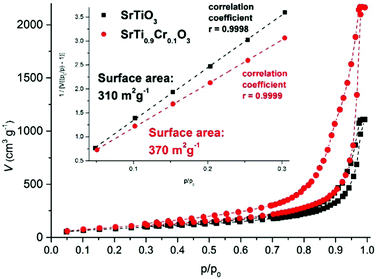
By nitrogen gas sorption experiments, the BET surface area and the porosity were determined (Fig. 6). For undoped SrTiO3 aerogels, the surface area measured 310 m2 g−1, whereas doping with Cr increased the surface area up to 370 m2 g−1 for 10% Cr, with the intermediate doping content lying in between these values (inset Fig. 6). The reason for this increase can be found in the quality of the dispersions, which improves through doping as mentioned above. This is also reflected in the pore size distribution in Fig. S7 (ESI†), as the 10% Cr doped sample shows a distinct pore size distribution with the majority of pores in the range of 20 nm, while the undoped sample shows a very broad distribution without a distinct pore size maximum. Samples doped with intermediate amounts lie in between these boundary values. As discussed above, SEM as local probe was obviously not able to resolve the minor microstructural differences between these samples. For all samples, no complete pore size distribution was observed, indicating the occurrence of large pores which are beyond the measurement range of nitrogen gas sorption, which is also reflected in the lack of a plateau at high relative pressures in the adsorption isotherm (Fig. 6). Electron microscopy techniques as in Fig. 5 however are well suited to cover this range of pore sizes. Other techniques such as mercury porosimetry are not suited for this kind of material, as the high pressures would significantly damage the aerogel microstructure. Compared to literature values, the surface areas measured in the present study of over 310 m2 g−1 are similar to those found for BaTiO339 and other metal oxide aerogels,48–50 and they are significantly higher than previous reports on SrTiO3 aerogels (130–159 m2 g−1),43,44 Cr doped SrTiO3 porous spheres (65.4 m2 g−1),25 Cr doped SrTiO3 nanotube architectures (40.5 m2 g−1),4 mesoporous SrTiO3 (24–28 m2 g−1)3,13 and hierarchically porous SrTiO3 calcined at 800 °C (2.9 m2 g−1).57
In sol–gel processing, another versatile use of nanoparticle dispersions is the production of thin films, which are used for many applications.58 In the present study, thin films were deposited on fused silica substrates by repeated dip coating of a 5% Cr doped SrTiO3 dispersion. The resulting homogeneous thin films showed a high transparency after deposition (Fig. 7a). SEM investigations revealed a smooth and dense thin film with a thickness of around 140 nm without occurrence of any large agglomerates or cracking as depicted in Fig. 8a. Upon heating to 600 °C, no macroscopic cracks or delamination was observed and the film still exhibited a high transparency (Fig. 7b). SEM images in Fig. 8b indicate a densification of the film to around 50 nm, corresponding to a thickness loss of ∼65%. Additionally, small surface defects (voids, cracks,…) occurred as a result of the shrinkage and the removal of organics from the film such as benzyl alcohol from synthesis and MEEAA from functionalization (Fig. 8c). However, the defects are homogeneously distributed over the entire sample and no significant cracking of the thin film is observed. This observation is remarkable, considering the large shrinking of the film thickness, while laterally no shrinkage occurs.
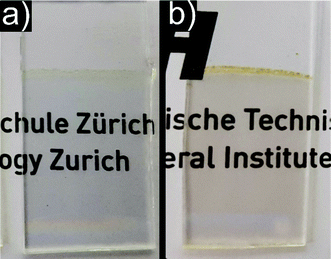 | ||
| Fig. 7 Photographs of a 5% Cr doped SrTiO3 thin film deposited on fused silica substrate by dip coating. (a) As deposited film and (b) heat treated film at 600 °C for 2 h in air. | ||
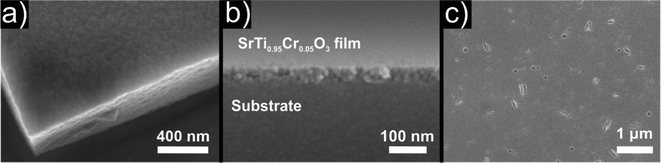 | ||
| Fig. 8 SEM images of 5% Cr doped SrTiO3 thin films (a) as deposited on the substrate, (b) cross section after annealing at 600 °C and (c) top view after annealing. | ||
Conclusion
A highly attractive feature of the wet-chemical processing of nanoparticle dispersions is the great flexibility to produce a nanostructured material of any desired shape including 3-dimensional monoliths or 2-dimensional films. In the present study, we made use of the high stability and high concentration of MEEAA functionalized SrTi1−xCrxO3 nanoparticles in ethanol dispersions. From these dispersions we were able to produce aerogels with high surface area (370 m2 g−1) through efficient destabilization and we deposited homogeneous thin films by simple dip coating. With the possibility to process doped SrTiO3 nanoparticles with its promising properties into 2- and 3-dimensional architectures, we set the basis for their efficient use in photocatalytic applications. As a matter of fact, the processing of nanoparticle powders into macroscopic bodies represents a major obstacle on the way to implement them in different applications and every example, which is able to overcome these challenges, brings us closer to this goal.Acknowledgements
Experimental assistance by Thomas Geldmacher and Meril Sindelar is acknowledged. We thank ETH Zurich and the Swiss National Science Foundation (Project No. 200021_165888) for financial support. The authors thank the Swiss Federal Laboratories for Materials Science and Technology (EMPA), Dubendorf, for providing the TEM facilities.References
- B. Jaffe, W. R. Cook Jr and H. Jaffe, Piezoelectric Ceramics, Academic Press, 1971, p. 185 Search PubMed.
- C. H. Ahn, K. M. Rabe and J.-M. Triscone, Science, 2004, 303, 488 CrossRef CAS PubMed.
- A. Bera, K. Wu, A. Sheikh, E. Alarousu, O. F. Mohammed and T. Wu, J. Phys. Chem. C, 2014, 118, 28494 CAS.
- D. Hou, X. Hu, W. Ho, P. Hu and Y. Huang, J. Mater. Chem. A, 2015, 3, 3935 CAS.
- K. Su, N. Nuraje and N.-L. Yang, Langmuir, 2007, 23, 11369 CrossRef CAS PubMed.
- Q.-S. Wu, J.-W. Liu, S.-F. Chen, H.-W. Liang and S.-H. Yu, CrystEngComm, 2015, 17, 6895 RSC.
- Y. Mao, S. Banerjee and S. S. Wong, Chem. Commun., 2003, 408 RSC.
- Y. Wang, G. Xu, L. Yang, Z. Ren, X. Wei, W. Weng, P. Du, G. Shen and G. Han, J. Cryst. Growth, 2009, 311, 2519 CrossRef CAS.
- D. Chen, X. Jiao and M. Zhang, J. Eur. Ceram. Soc., 2000, 20, 1261 CrossRef CAS.
- S. Fuentes, R. A. Zarate, E. Chavez, P. Muñoz, D. Díaz-Droguett and P. Leyton, J. Mater. Sci., 2010, 45, 1448 CrossRef CAS.
- W. Xuewen, Z. Zhiyong and Z. Shuixian, Mater. Sci. Eng., B, 2001, 86, 29 CrossRef.
- H. Cui, M. Zayat and D. Levy, J. Non-Cryst. Solids, 2007, 353, 1011 CrossRef CAS.
- T. Puangpetch, T. Sreethawong, S. Yoshikawa and S. Chavadej, J. Mol. Catal. A: Chem., 2008, 287, 70 CrossRef CAS.
- P. Visuttipitukul, P. Sooksaen and N. Yongvanich, Ferroelectrics, 2013, 457, 82 CrossRef CAS.
- J. Wang, S. Yin, M. Komatsu, Q. Zhang, F. Saito and T. Sato, J. Photochem. Photobiol., A, 2004, 165, 149 CrossRef CAS.
- W. Wei, Y. Dai, M. Guo, L. Yu and B. Huang, J. Phys. Chem. C, 2009, 113, 15046 CAS.
- R. Konta, T. Ishii, H. Kato and A. Kudo, J. Phys. Chem. B, 2004, 108, 8992 CrossRef CAS.
- V. Subramanian, R. K. Roeder and E. E. Wolf, Ind. Eng. Chem. Res., 2006, 45, 2187 CrossRef CAS.
- T.-H. Xie, X. Sun and J. Lin, J. Phys. Chem. C, 2008, 112, 9753 CAS.
- L. Chen, S. Zhang, L. Wang, D. Xue and S. Yin, J. Cryst. Growth, 2009, 311, 735 CrossRef CAS.
- C.-H. Chang and Y.-H. Shen, Mater. Lett., 2006, 60, 129 CrossRef CAS.
- S. Tonda, S. Kumar, O. Anjaneyulu and V. Shanker, Phys. Chem. Chem. Phys., 2014, 16, 23819 RSC.
- J. W. Liu, G. Chen, Z. H. Li and Z. G. Zhang, J. Solid State Chem., 2006, 179, 3704 CrossRef CAS.
- T. Ishii, H. Kato and A. Kudo, J. Photochem. Photobiol., A, 2004, 163, 181 CrossRef CAS.
- H. Yu, S. Ouyang, S. Yan, Z. Li, T. Yu and Z. Zou, J. Mater. Chem., 2011, 21, 11347 RSC.
- D. Wang, J. Ye, T. Kako and T. Kimura, J. Phys. Chem. B, 2006, 110, 15824 CrossRef CAS PubMed.
- H. Kato and A. Kudo, J. Phys. Chem. B, 2002, 106, 5029 CrossRef CAS.
- P. Reunchan, N. Umezawa, S. Ouyang and J. Ye, Phys. Chem. Chem. Phys., 2012, 14, 1876 RSC.
- V. A. Trepakov, Z. Potůček, M. V. Makarova, A. Dejneka, P. Sazama, L. Jastrabik and Z. Bryknar, J. Phys.: Condens. Matter, 2009, 21, 375303 CrossRef CAS PubMed.
- M. S. Wrighton, A. B. Ellis, P. T. Wolczanski, D. L. Morse, H. B. Abrahamson and D. S. Ginley, J. Am. Chem. Soc., 1976, 98, 2774 CrossRef CAS.
- D. Sui, X. Yin, H. Dong, S. Qin, J. Chen and W. Jiang, Catal. Lett., 2012, 142, 1202 CrossRef CAS.
- Y. Bi, M. F. Ehsan, Y. Huang, J. Jin and T. He, J. CO2 Util., 2015, 12, 43 CrossRef CAS.
- H. D. Meierling, Phys. Status Solidi, 1971, 43, 191 CrossRef.
- G. I. M. M. Janousch, U. Staub, B. Delley, S. F. Karg and B. P. Andreasson, 2007, arXiv:0707.0563 [cond-mat.mtrl-sci].
- F. La Mattina, J. G. Bednorz, S. F. Alvarado, A. Shengelaya and H. Keller, Appl. Phys. Lett., 2008, 93, 022102 CrossRef.
- D. R. Rolison, Science, 2003, 299, 1698 CrossRef CAS PubMed.
- M. Niederberger, G. Garnweitner, N. Pinna and M. Antonietti, J. Am. Chem. Soc., 2004, 126, 9120 CrossRef CAS PubMed.
- M. Niederberger, N. Pinna, J. Polleux and M. Antonietti, Angew. Chem., 2004, 116, 2320 CrossRef.
- F. Rechberger, F. J. Heiligtag, M. J. Süess and M. Niederberger, Angew. Chem., Int. Ed., 2014, 53, 6823 CrossRef CAS PubMed.
- D. Erdem, Y. Shi, F. J. Heiligtag, A. C. Kandemir, E. Tervoort, J. L. M. Rupp and M. Niederberger, J. Mater. Chem. C, 2015, 3, 9833 RSC.
- F. Rechberger, C. Mercandetti, E. Tervoort and M. Niederberger, Langmuir, 2017, 33, 280 CrossRef CAS PubMed.
- F. Rechberger and M. Niederberger, Nanoscale Horiz., 2017, 2, 6 RSC.
- D. Demydov and K. J. Klabunde, J. Non-Cryst. Solids, 2004, 350, 165 CrossRef CAS.
- K. Yenting and J. K. Kenneth, Nanotechnology, 2012, 23, 294001 CrossRef PubMed.
- J. L. Mohanan, I. U. Arachchige and S. L. Brock, Science, 2005, 307, 397 CAS.
- R. Deshmukh, E. Tervoort, J. Kach, F. Rechberger and M. Niederberger, Dalton Trans., 2016, 45, 11616 RSC.
- N. C. Bigall, A.-K. Herrmann, M. Vogel, M. Rose, P. Simon, W. Carrillo-Cabrera, D. Dorfs, S. Kaskel, N. Gaponik and A. Eychmüller, Angew. Chem., Int. Ed., 2009, 48, 9731 CrossRef CAS PubMed.
- F. J. Heiligtag, M. D. Rossell, M. J. Süess and M. Niederberger, J. Mater. Chem., 2011, 21, 16893 RSC.
- F. Rechberger, G. Ilari and M. Niederberger, Chem. Commun., 2014, 50, 13138 RSC.
- W. Cheng, F. Rechberger and M. Niederberger, ACS Nano, 2016, 10, 2467 CrossRef CAS PubMed.
- W. Cheng, F. Rechberger and M. Niederberger, Nanoscale, 2016, 8, 14074 RSC.
- D. A. Prystupa, A. Anderson and B. H. Torrie, J. Raman Spectrosc., 1994, 25, 175 CrossRef CAS.
- D. Amans, C. Malaterre, M. Diouf, C. Mancini, F. Chaput, G. Ledoux, G. Breton, Y. Guillin, C. Dujardin, K. Masenelli-Varlot and P. Perriat, J. Phys. Chem. C, 2011, 115, 5131 CAS.
- T. A. Cheema and G. Garnweitner, CrystEngComm, 2014, 16, 3366 RSC.
- D. W. Hwang, H. G. Kim, J. S. Lee, J. Kim, W. Li and S. H. Oh, J. Phys. Chem. B, 2005, 109, 2093 CrossRef CAS PubMed.
- F. Rechberger, R. Städler, E. Tervoort and M. Niederberger, J. Sol-Gel Sci. Technol., 2016, 80, 660 CrossRef CAS.
- O. Ruzimuradov, G. Hasegawa, K. Kanamori and K. Nakanishi, J. Am. Ceram. Soc., 2011, 94, 3335 CrossRef CAS.
- M. Niederberger and N. Pinna, Metal Oxide Nanoparticles in Inorganic Solvents – Synthesis, Formation, Assembly and Application, Springer, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, XRD Rietveld refinement and unit cell parameters, IR spectra, TEM overview, EDX spectra and mapping, DFT pore size distribution. See DOI: 10.1039/c7qm00155j |
| This journal is © the Partner Organisations 2017 |