Hybrid polymeric nanoprobes for folate receptor-targeted photoacoustic imaging in vivo†
Yihong
Li‡
ab,
Yuanhui
Song‡
ab,
Waner
Chen
ab,
Qien
Xu
ab and
Zhe
Liu‡
*ab
aWenzhou Institute of Biomaterials and Engineering, Wenzhou Medical University, Wenzhou 325011, Zhejiang, China. E-mail: liuzhe@wibe.ac.cn
bWenzhou Institute of Biomaterials and Engineering, Chinese Academy of Sciences, Wenzhou 325001, Zhejiang, China
First published on 7th December 2016
Abstract
To construct biocompatible imaging nanoprobes and apply them to receptor-specific targeting of breast cancers is of great significance for precise diagnosis and personalized therapy serving nanomedicine. Herein, hybrid polymeric methylene blue-embedded nanoprobes (PBCA-MB@CS-FA) for folate receptor-targeted photoacoustic (PA) imaging have been successfully fabricated. Phantom imaging and in vitro cell experiments confirmed their apparent contrast enhancement and strong binding affinity. The administration into MDA-MB231 tumor-xenografted nude mice showed 10-fold higher intensity of PA signals compared to that of non-targeted control. These features make them promising PA nanoprobes for non-invasive molecular diagnosis, and more importantly for potential PA image-guided surgery/therapy against breast cancers in the early stage.
Breast cancer is one of the most common malignant diseases especially in women with an incidence rate of 12.4%.1,2 Thus, a successful strategy for early detection and precise diagnosis is vitally important for the treatment and final recovery of patients.3,4 At present, numerous diagnostic techniques including biomedical imaging at the cellular and molecular levels have been widely used for breast cancer determination. Mammography has been regarded as a standard screening method for breast cancer.5 However, due to radiological risks and inadequate positioning for retromammary tumors, this technique can only be roughly utilized for primary assessment. Magnetic resonance imaging (MRI) has been recommended by the American Cancer Society (ACS) to scan for high-risk breast lesions with satisfactory sensitivity.6 Nevertheless, high cost and time-consuming imaging have become major obstacles for MRI. In contrast, ultrasound is an attractive supplement because of several prominent features e.g. non-ionization, ready availability, low cost and acceptable tolerance by patients.7,8 Moreover, in order to enhance the diagnostic precision, photoacoustic (PA) imaging can be used by combining both optical high sensitivity and acoustic deep penetration.9,10 For instance, photoacoustic endoscopy has been used for diagnosing superficial lesions (e.g. breast and melanoma tumors) and determination of tumor infiltration depth (e.g. oesophagus and colon cancer staging) with the endogenous contrast enhancement from hemoglobin, melanin and lipids in tissues.11 On the other hand, exogenous PA imaging probes may enable not only biomarker-targeted binding but also active tumor accumulation to serve molecular diagnosis and effective therapy of breast cancers with higher specificity and better image resolution.12–14 Optically absorbing fluorescent dyes, plasmonic gold nanoparticles, carbon nanotubes and biophotonic nanoparticles have been developed as exogenous PA contrast agents since they are excited in the near-infrared range, exhibit a strong PA effect over endogenous substances and thus provide better contrast enhancement. Biological ligands targeting specific biomarkers overexpressed on breast tumors can be conjugated with PA probes so that they can be exploited to differentiate normal and diseased tissues and work for breast angiogenesis imaging, tumor boundary determination and PA image-guided surgery.15–26 Gold nanorods with paclitaxel (PTX) and perfluorohexane (PFH) loading have been developed for the induction of apoptosis of cancer cells.22 However, the biosafety of gold nanoparticles is still a big issue for successful clinical translation to humans.14,17,23 Indocyanine green-labeled single walled nanotube (ICG-SWNT) conjugates have been proposed as tumor-targeted PA probes which could yield a significant increase in the tumor-to-background ratio by 200%.15 However, the clinical applications of SWNTs have not been conducted yet because of the poor degradation performance which probably leads to long-term toxicity in organs such as liver, lung, and spleen.24
Therefore, fabricating PA probes with biocompatible materials and clinically approved near-infrared dyes is of great importance not only for fundamental research but also for clinical translations. As a clinical PA contrast agent, methylene blue (MB) has gained emerging attention owing to their unique biocompatibility, safety and low-cost.27,28 It is currently utilized as a PA tracer to localize sentinel lymph nodes accurately.29 In addition, it also acts as a dual-functional probe for the simultaneous PA-US imaging of breast cancer efficiently.30 However, rapid body-clearance of MB has been a major limitation for direct applications to sustained imaging and collecting pathogenic information. Poly butyl cyanoacrylate (PBCA) has been reported as a biocompatible polymer which is mainly utilized for drug delivery systems, ultrasound imaging and regenerative medicine.31–33 To construct a hybrid PBCA-based MB-embedded PA probe will definitely improve the clearance properties in vivo, optimize the PA imaging performance and also endow targeting capability to breast tumors. Herein, we report the synthesis of the hybrid PBCA-MB nanoparticles via monomer emulsification and in situ MB encapsulation. By electrostatic interactions, chitosan (CS) as a natural biomaterial was selected to surface-coat PBCA-MB and achieve positively charged PBCA-MB@CS. Folic acid (FA), a high-affinity ligand to folate receptors (FRs) overexpressed in most breast cancer cells, has been reported to serve PA imaging and photothermal therapy simultaneously.34–38 In this study, FA was conjugated to afford PBCA-MB@CS-FA as novel targeting nanoprobes for PA imaging of breast tumors in vivo (Fig. 1). It proved that the as-fabricated hybrid FR-targeted PA nanoprobes could specifically bind the breast cancer cells MDA-MB231 with high affinity in vitro which has been verified using confocal laser scanning microscopy (CLSM) and flow cytometry. In vivo PA imaging after injection of PBCA-MB@CS-FA into MDA-MB231 tumor-xenografted nude mice displayed obvious tumor accumulation up to 24 h and sustainable significant PA contrast enhancement compared to the control models. These exciting findings evidenced that these hybrid polymeric MB-embedded nanoprobes are not only promising for in vivo PA molecular imaging but also provide an unprecedented opportunity for non-invasive molecular diagnosis and potential image-guided targeting therapy in the early stage of breast cancers.
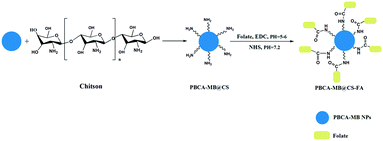 | ||
| Fig. 1 Illustrative fabrication of hybrid PBCA-MB@CS-FA nanoprobes using emulsion polymerization, chitosan surface coating and folic acid bio-conjugation. | ||
First of all, butyl cyanoacrylate as a monomer was reacted under acidic conditions associated with methylene blue embedding to afford PBCA-MB nanoparticles in a one-pot manner. By dynamic light scattering (DLS), PBCA-MB were characterized to be 530.0 ± 0.2 nm with negative charges (−40.0 ± 0.2 mV) on the surface. Compared to bare PBCA, the size of PBCA-MB dramatically increased along with more negative charges (see the ESI,† Table S1). This might be explained by the good hydrophilicity of MB that leads to effective encapsulation of massive MB within the PBCA polymer. Then chitosan as a polycationic and biocompatible polymer was chosen to surface-coat PBCA-MB and realize a positive charge conversion. In the meantime, the introduction of chitosan ensures the following FA conjugation by providing adequate amino groups on the particle surface. The as-prepared PBCA-MB@CS was reacted with FA via a traditional EDC–NHS activation–conjugation protocol to yield PBCA-MB@CS-FA.39
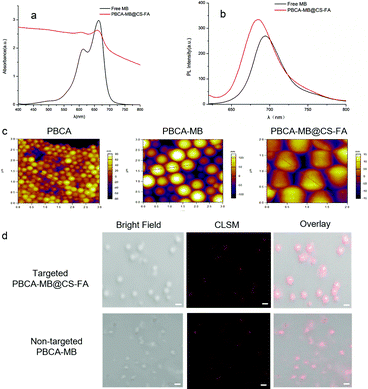
To validate that MB has been successfully encapsulated, UV-Vis absorption and photoluminescence (PL) emission spectra were acquired. As shown in Fig. 2a, PBCA-MB@CS-FA displayed identical characteristic absorption peaks at wavelengths of 615 nm and 680 nm as measured with free MB although the absorption intensity was relatively low. Besides, PBCA-MB@CS-FA represented a similar PL emission spectrum in the near-infrared region as compared with free MB in spite of a slight blue-shift due to the internal MB encapsulation (Fig. 2b). These results reflected that the encapsulation of MB in the hybrid PBCA-MB@CS-FA nanoprobes had been accomplished and that this did not influence their optical properties. The MB encapsulation efficiency was determined to be 6.4 ± 0.7% by triplicate UV-Vis spectrophotometry. Next, atomic force microscopy (AFM) was applied to measure the sizes of PBCA, PBCA-MB and PBCA-MB@CS-FA, and these data were in good accordance with DLS analysis (Fig. 2c and Fig. S1 in the ESI†). To directly verify their sizes and prove the embedded MB distribution, CLSM were used. The estimation of PBCA-MB and PBCA-MB@CS-FA sizes were in agreement with those by AFM and DLS as previously mentioned. Bright field, CLSM and merged images all convinced that hybrid nanoprobes PBCA-MB@CS-FA were fabricated, and that the MB was uniformly embedded inside (Fig. 2d).
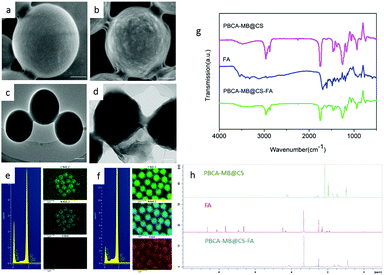
To further investigate their morphology and chemical characterization, PBCA-based nanoparticles were analyzed using electron microscopy, FT-IR and NMR (Fig. 3). Scanning electron microscopy (SEM) was performed to examine the surface morphology between PBCA-MB and PBCA-MB@CS-FA, and it was obviously shown that PBCA-MB had a smooth surface whereas PBCA-MB@CS-FA displayed a relatively rough and viscous surface probably due to the covalent formation of amides and FA anchoring (Fig. 3a and b). This was also found in transmission electron microscopy (TEM) images that PBCA-MB showed a solid and uniform spherical structure, but PBCA-MB@CS-FA exhibited a suppressed structure and adhered to the surrounding particles (Fig. 3c and d). Energy dispersive spectroscopy (EDS) was also performed and complementarily proved that the composition of C, N and O slightly increased for PBCA-MB@CS-FA due to chitosan coating and folic acid conjugation (Fig. 3e and f). Moreover, FT-IR and NMR were exploited to identify the existence of amides and the conjugation of folic acids. A broad absorption band at 3200–3400 cm−1 was recognized as the characteristic vibration of primary amine (–NH2) and secondary amide (–NH–), which resulted from the chitosan coating and folic acid conjugation (Fig. 3g). Pteridine aromatic vibration peaks in the range of 1478–1694 cm−1 and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration at 1703 cm−1 provided further supporting evidence of the folic acid structure. In addition, the NMR data of PBCA-MB@CS-FA also gave the corresponding signals of folic acid building blocks in the low field (6.64, 6.93, 7.65, 8.12, 8.64, 11.4 ppm) which drew the conclusion that folic acid has been conjugated to afford the desired targeted PBCA-MB@CS-FA agents (Fig. 3h and Fig. S2 in the ESI†). The conjugation quantity of the folic acids was determined to be 8.31 mg mL−1 by triplicate measurements of OD values using UV-Vis spectrophotometry at a wavelength of 281 nm, which was the maximal absorption peak of folic acid molecules.
O stretching vibration at 1703 cm−1 provided further supporting evidence of the folic acid structure. In addition, the NMR data of PBCA-MB@CS-FA also gave the corresponding signals of folic acid building blocks in the low field (6.64, 6.93, 7.65, 8.12, 8.64, 11.4 ppm) which drew the conclusion that folic acid has been conjugated to afford the desired targeted PBCA-MB@CS-FA agents (Fig. 3h and Fig. S2 in the ESI†). The conjugation quantity of the folic acids was determined to be 8.31 mg mL−1 by triplicate measurements of OD values using UV-Vis spectrophotometry at a wavelength of 281 nm, which was the maximal absorption peak of folic acid molecules.
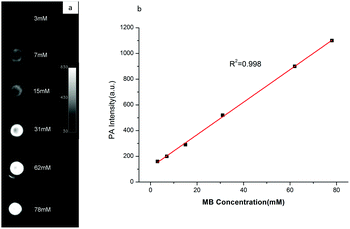
In order to test the availability of the hybrid PBCA-MB@CS-FA nanoprobes for PA imaging, phantom imaging experiments were accordingly performed. In a photoacoustic field, a series of PBCA-MB@CS-FA samples with different embedded MB concentrations (3–78 mM) were excited by a laser at a wavelength of 680 nm, an ultrasound transducer was fixed over the samples to receive the photoacoustic signals. PA images were then reconstructed and displayed so that the signal intensity was closely dependent on the MB concentration in PBCA-MB@CS-FA (Fig. 4a). An obvious PA contrast could be observed when the MB concentration was as low as 7 mM, and a strong contrast enhancement was visualized when the MB concentration was over 31 mM. A linear intensity-concentration correlation was also acquired (R2 = 0.998) (Fig. 4b). These exciting findings demonstrated that the hybrid nanoprobes PBCA-MB@CS-FA could provide PA images with high sensitivity and satisfactory contrast enhancement even at very low concentrations. In comparison to common PA agents, they displayed comparable contrast enhancement,15,36,38 which encourages us to further study the feasibility for in vitro cell targeting and in vivo small-animal PA imaging for breast cancers.
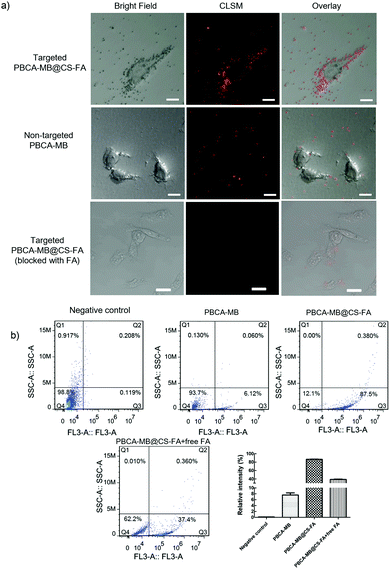
Considering that folate receptors are generally overexpressed on breast cancer cell membranes,34–40 FR-targeted and non-targeted PBCA-based nanoprobes were exploited for in vitro cell targeting studies. Human breast cell MDA-MB231 was selected as an FR-positive (FR+) cell line for incubation with FR-targeted PBCA-MB@CS-FA and non-targeted PBCA-MB, respectively. In the meantime, human glioblastoma cells U87MG were chosen as the FR-negative control (FR−) cell line for the same targeting test. After incubation with the targeted nanoprobes, different cell lines were located on a confocal laser scanning microscope to visualize the targeting efficiency. As shown in Fig. 5a, MDA-MB231 treated with targeted PBCA-MB@CS-FA displayed excellent FR-targeting capabilities, and a great deal of red fluorescent spots (i.e. PBCA-MB@CS-FA) were observed binding to the cell membranes which confirmed their specific attachment to the breast cancer cells. Besides that, low red fluorescence could also be visualized inside the cells probably due to receptor-mediated endocytosis (RME). To further identify the FR-targeting profile of the nanoprobes, free FA was co-incubated with MDA-MB231 cells to compete with the binding of PBCA-MB@CS-FA. It was found that after blocking of free FA, less PBCA-MB@CS-FA was observed attached to the MDA-MB231 cells. This consolidated the conclusion that the FR-targeting mechanism enables the targeted PBCA-MB@CS-FA to bind the FA receptors overexpressed on breast cancer cells, and just a low concentration of embedded MB endows the high sensitivity for image differentiation. As a control, non-targeted PBCA-MB hardly showed interactions to MDA-MB231 except that only free isolated PBCA-MB nanoparticles remained in the cell culture medium. U87MG treated with targeted PBCA-MB@CS-FA was also examined using CLSM (Fig. S3 in the ESI†), and they exhibited no targeting effect.
In order to further quantitatively evaluate the targeting capability, flow cytometry was carried out by counting the MDA-MB231 cells labelled by PBCA-MB@CS-FA after co-incubation (Fig. 5b). It was found that 87.5% of the MDA-MB231 cells were labelled by PBCA-MB@CS-FA, while only 6.1% were labelled by PBCA-MB and 0.1% of U87MG were labelled by PBCA-MB@CS-FA. In the competition experiments, the relative intensity was 38.8% (FA blocking) compared to 87.5% (non-blocking), which indicated that due to free FA blocking, the targeting of PBCA-MB@CS-FA to MDA-MB231 has been dramatically inhibited. These in vitro cell-targeting data proved that the hybrid nanoprobes PBCA-MB@CS-FA exhibited significant targeting capabilities and ensured themselves eligible for FR-targeted PA imaging in vivo.
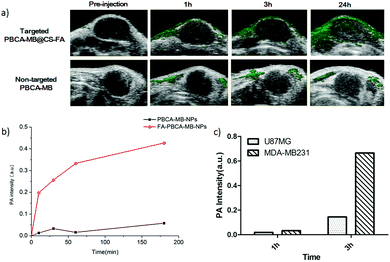
The in vivo PA imaging for breast cancer targeting was performed by using MDA-MB231 tumor-xenografted nude mice as FR+ animal models and U87MG tumor-bearing nude mice as the control. Targeted PBCA-MB@CS-FA and non-targeted PBCA-MB were subcutaneously injected close to the tumors. Merged PA and ultrasound images were acquired at different time points through 24 h to monitor their targeting, record tumor accumulation and position the tumor boundaries. At 1 h post-injection, the MDA-MB231 tumor-bearing nude mice that were injected with targeted PBCA-MB@CS-FA demonstrated enhanced PA contrast in the tumor region compared to the non-targeted PBCA-MB (Fig. 6a). Peripheral areas around the tumor also displayed PA contrast to some extent which might be due to the probe diffusion into the tissues. At 3 h and 24 h post-injection, PA signals were recorded dramatically increasing as a result of the gradual accumulation of targeted nanoprobes PBCA-MB@CS-FA into the tumor and specific binding to the folate receptors overexpressed on the MDA-MB231 cells. However, no significant PA signal enhancement was captured within the tumor when non-targeted PBCA-MB was administered throughout 24 h (Fig. 6a).
To make detailed investigations of in vivo PA imaging, quantitative analysis on the PA signal intensity was afforded. The PA signal in the MDA-MB231 tumor administrated with PBCA-MB@CS-FA increased significantly at 10 min, and the increase was constantly maintained till 24 h post-injection (red curve in Fig. 6b). In contrast, although an obscure increase in PA signals was also observed at 30 min post-injection of non-targeted PBCA-MB possibly due to nanoprobe retention/blocking, the intensity rapidly decreased and the overall change throughout 24 h was negligible (black curve in Fig. 6b). Recently a study showed that the PA signal enhancement in the tumor region was 6-fold higher by using FR-targeted nanoparticles.36 In comparison, our present study demonstrated that at 3 h post-injection of targeted PBCA-MB@CS-FA, the PA signal intensity in the MDA-MB231 tumor was 10-fold higher than that in U87MG, indicating that targeted nanoprobes produced more persistent and efficient PA contrast enhancement (Fig. 6c). When targeted PBCA-MB@CS-FA and non-targeted PBCA-MB were injected into the U87MG tumor-bearing nude mice respectively, a faint increase was recorded at 30 min, but afterwards it fell down which implied a fast clearance (Fig. S4a and b in the ESI†). These results concluded that the hybrid FR-targeted nanoprobes are prominent for non-invasive PA molecular imaging as well as potential PA image-guided treatment against breast cancers in the early stage.
In conclusion, novel hybrid polymeric methylene blue-embedded nanoprobes (PBCA-MB@CS-FA) for folate receptor-targeted PA molecular imaging have been successfully fabricated. Optical, structural and chemical characterizations have been well studied. Phantom imaging and in vitro cell experiments confirmed that these FR-targeted nanoprobes exhibited apparent contrast enhancement and strong binding affinity to breast cancer cells MDA-MB231. Their administration into MDA-MB231 tumor-bearing nude mice showed 10-fold higher intensity of PA signals compared to that of the non-targeted control. These findings demonstrate that they are not only promising for non-invasive molecular diagnosis, but also provide unprecedented choices for potential PA image-guided surgery/therapy in the early stage of breast cancers.
This work was financially supported by the National Natural Science Foundation of China (21575106), the Scientific Research Foundation for Returned Scholars, the Ministry of Education of China, Zhejiang Qianjiang Talents Program and Wenzhou Government's Start-up Fund.
Notes and references
- H. Seidman, S. D. Stellman and M. H. Mushinski, Ca-Cancer J. Clin., 1982, 32, 301–313 CrossRef CAS PubMed.
- S. E. Jackson and J. D. Chester, Int. J. Cancer, 2015, 137, 262–266 CrossRef CAS PubMed.
- C. I. Lee, L. S. Gold, H. D. Nelson, R. Chou and S. D. Ramsey, Breast, 2015, 24, 3–11 CrossRef PubMed.
- T. Faruk, M. K. Islam, S. Arefin and M. Z. Hag, Clin. Breast Cancer, 2015, 15, 313–324 CrossRef PubMed.
- D. G. Evans and A. J. Maxwell, J. Natl. Cancer Inst., 2016, 38, 108 Search PubMed.
- E. C. Gombos, J. Jaqadeesan, D. M. Richman and D. F. Kacher, Magn. Reson. Imaging Clin. N. Am., 2015, 23, 547–561 CrossRef PubMed.
- S. Tardoski, J. Ngo, E. Gineytes, J. P. Roux and P. Clezardin, Sci. Rep., 2015, 5, 16354 CrossRef CAS PubMed.
- A. Copelan, J. Hartman, M. Chehab and A. M. Venkatesan, Intervent. Radiol., 2015, 32, 398–415 CrossRef PubMed.
- S. Zackrisson, S. M. van de Ven and S. S. Gambhir, Cancer Res., 2014, 74, 979–1004 CrossRef CAS PubMed.
- J. Yao and L. V. Wang, Contrast Media Mol. Imaging, 2011, 6, 332–345 CrossRef CAS PubMed.
- T. J. Yoon and Y. S. Cho, World J. Gastrointest. Endosc., 2013, 5, 534–539 CrossRef PubMed.
- G. P. Luke, D. Yeager and S. Y. Emelianov, Ann. Biomed. Eng., 2012, 40, 422–437 CrossRef PubMed.
- K. Li and B. Liu, Chem. Soc. Rev., 2014, 43, 6570–6597 RSC.
- W. Li and X. Chen, Nanomedicine, 2015, 10, 299–320 CrossRef CAS PubMed.
- A. de la Zerda, Z. Liu, S. Bodapati, R. Teed and S. Vaithilingam, Nano Lett., 2010, 10, 2168–2172 CrossRef CAS PubMed.
- H. F. Zhang, K. Maslov, G. Stoica and L. V. Wang, Nat. Biotechnol., 2016, 24, 848–851 CrossRef PubMed.
- J. Zhong, L. Wen, S. Yang, L. Xiang, Q. Chen and X. Ding, Nanomedicine, 2015, 11, 1499–1509 CAS.
- T. Bao, W. Yin, X. Zheng, X. Zhang, J. Yu, X. Dong, Y. Yong, F. Gao, L. Yan, Z. Gu and Y. Zhao, Biomaterials, 2016, 76, 11–26 CrossRef CAS PubMed.
- M. Zhou, B. Singhana, Y. Liu, Q. Huang, T. Mitcham, M. J. Wallace, R. J. Stafford, R. R. Bouchard and M. P. Melancon, J. Biomed. Nanotechnol., 2015, 11, 1442–1450 CrossRef CAS PubMed.
- L. Cheng, J. Liu, X. Gu, H. Gong, X. Shi, T. Liu, C. Wang, X. Wang, G. Liu, H. Xing, W. Bu, B. Sun and Z. Liu, Adv. Mater., 2014, 26, 1886–1893 CrossRef CAS PubMed.
- K. Pu, A. J. Shuhendler, J. V. Jokerst, J. Mei, S. S. Gambhir, Z. Bao and J. Rao, Nat. Nanotechnol., 2014, 9, 233–239 CrossRef CAS PubMed.
- J. Z. Zhong, S. H. Yang, L. W. Wen and D. Xing, J. Controlled Release, 2016, 226, 77–87 CrossRef CAS PubMed.
- Y. Liu, J. He, K. Yang, C. Yi, Y. Liu, L. Nie, N. M. Khashab, X. Chen and Z. Nie, Angew. Chem., Int. Ed., 2015, 54, 15809–15812 CrossRef CAS PubMed.
- S. T. Yang, X. Wang, G. Jia, Y. Gu, T. Wang, H. Nie, C. Ge, H. Wang and Y. Liu, Toxicol. Lett., 2008, 181, 182–189 CrossRef CAS PubMed.
- E. Huynk and G. Zheng, IEEE J. Sel. Top. Quantum Electron., 2014, 20, 27–34 CrossRef.
- H. D. Lu, B. K. Wilson, A. Heinmiller, B. Faenza, S. Hejazi and R. K. Prudhomme, ACS Appl. Mater. Interfaces, 2016, 8, 14379–14388 Search PubMed.
- M. Jeon, W. Song, E. Huynh, J. Kim, J. Kim, B. L. Helfield, B. Y. Leung, D. E. Goertz, G. Zheng, J. Oh, J. F. Lovell and C. Kim, J. Biomed. Opt., 2014, 19, 16005 CrossRef PubMed.
- E. Morgounova, Q. Shao, B. J. Hackel, D. D. Thomas and S. Ashkenazi, J. Biomed. Opt., 2013, 18, 56004 CrossRef PubMed.
- K. H. Song, E. W. Stein, J. A. Margenthaler and L. V. Wang, J. Biomed. Opt., 2008, 13, 54033 CrossRef PubMed.
- C. Kim, T. N. Erpelding, L. Jankovic and L. V. Wang, Philos. Trans. R. Soc., A, 2011, 369, 4644–4650 CrossRef PubMed.
- Y. Morch, R. Hansen, S. Berg, A. K. Aslund, W. R. Glomm, S. Eggen, R. Schmid, H. Johnsen, S. Kubowicz, S. Snipstad, E. Sulheim, S. Hak, G. Singh, B. H. McDonagh, H. Blom, C. de Lange Davies and P. M. Stenstad, Contrast Media Mol. Imaging, 2015, 10, 356–366 CrossRef CAS PubMed.
- Z. Liu, T. Lammers, J. Ehling, S. Fokong, J. Bornemann, F. Kiessling and J. Gaetjens, Biomaterials, 2011, 32, 6155–6163 CrossRef CAS PubMed.
- Z. Liu, P. Koczera, D. Doleschel, F. Kiessling and J. Gaetjens, Chem. Commun., 2012, 48, 5142–5144 RSC.
- Y. Zhang, J. M. Liu and X. P. Yan, Anal. Chem., 2013, 85, 228–234 CrossRef CAS PubMed.
- L. C. Hartmann, G. L. Keeney, W. L. Lingle, T. J. H. Christianson, B. Varghese, D. Hillman, A. L. Oberg and P. S. Low, Int. J. Cancer, 2007, 121, 938–942 CrossRef CAS PubMed.
- H. Wang, C. Liu, X. Gong, D. Hu, R. Lin, Z. Sheng, C. Zheng, M. Yan, J. Chen, L. Cai and L. Song, Nanoscale, 2014, 6, 14270–14279 RSC.
- A. R. Hilgenbrink and P. S. Low, J. Pharm. Sci., 2005, 94, 2135–2146 CrossRef CAS PubMed.
- J. Wang, R. Yan, F. Guo, M. Yu, F. Tan and N. Li, Nanotechnology, 2016, 27, 285102 CrossRef PubMed.
- G. T. Hermanson, Bioconjugate Techniques, Academic Press, 2nd edn, 2013 Search PubMed.
- S. Setua, D. Menon, A. Asok, S. Nair and M. Koyakutty, Biomaterials, 2010, 31, 714–729 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, including fabrication and characterization of PBCA-based nanoprobes, and the protocols for cell targeting, flow cytometry and in vivo PA imaging of nude mice. See DOI: 10.1039/c6qm00227g |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2017 |