Facile solvothermal synthesis and superior lithium storage capability of Co3O4 nanoflowers with multi-scale dimensions†
Bin
Wang
*b,
Xiao-Ying
Lu
*a,
King Yan
Wong
a and
Yuanyuan
Tang
c
aFaculty of Science and Technology, Technological and Higher Education Institute of Hong Kong, Hong Kong, P. R. China. E-mail: xylu@vtc.edu.hk; Fax: +852-21761554; Tel: +852-21761453
bHong Kong Applied Science and Technology Research Institute, Hong Kong, P. R. China. E-mail: hku507@gmail.com; bwang@astri.org; Fax: +852-34062802; Tel: +852-34062561
cSchool of Environmental Science and Engineering, South University of Science and Technology of China, Shenzhen, 518055, P. R. China
First published on 18th August 2016
Abstract
In this study, Co3O4 nanoflowers (Co3O4-NFs) have been successfully synthesized by a facile ammonia-assisted solvothermal process and subsequent heat treatment. By suitably increasing ammonia solution added during solvothermal synthesis, flower-like Co3O4 structures were tailored from nanoflowers to microflowers (Co3O4-MFs). Materials characterization indicated that Co3O4-NFs with a specific surface area as high as 103.9 m2 g−1 were composed of multi-scale dimensions, including nanoparticles (0D) of about 10 nm in size, nanosheets (2D) of about 10 nm in thickness and nanoflowers (3D) of 290 ± 25 nm in diameter. The unique structural characteristics were beneficial for fast lithium ion diffusion and efficient electron transport. In addition, the mesoporous structure (22.6 nm) and the large pore volume (0.587 cm3 g−1) of Co3O4-NFs were favorable for effectively alleviating volume expansion problems of high-capacity anode materials during charge–discharge cycles. When Co3O4-NFs were investigated as anode materials for lithium ion batteries, superior lithium storage capability, excellent cycling stability and C-rate performance were achieved, in comparison with Co3O4-MFs and commercial Co3O4 micro-/nanoparticles (Co3O4-NPs). Specifically, when tested at a current density of 500 mA g−1 for 100 cycles, the reversible specific capacity of 1323 mA h g−1 was achieved for Co3O4-NFs, much higher than Co3O4-MFs (1281 mA h g−1) and Co3O4-NPs (107 mA h g−1). When current densities of C-rate tests were increased to 1000, 2000 and 3000 mA g−1, Co3O4-NFs could still deliver average specific capacities of around 1081, 925.9 and 570.8 mA h g−1, respectively. Owing to the intriguing merits from the unique characteristics, Co3O4-NFs with a nanoflower structure could achieve remarkable lithium storage capability, demonstrating great potential in next-generation lithium ion batteries.
Introduction
Rechargeable lithium ion batteries (LIBs) have achieved great success in portable electronic devices, such as cell phones, digital cameras and notebooks, owing to their high energy density, memory-free effect, long life span and environmental benignity.1–5 Currently, LIBs also have promising widespread applications in hybrid electric vehicles (HEVs) and electric vehicles (EVs) to improve roadside air quality by reducing the emission of fine particulate matter (e.g. PM 2.5) and various types of greenhouse gases (e.g. CO2 and NOx). However, with the ever-increasing demands in energy density, high performance LIBs have encountered technical challenges for future applications in HEVs and EVs. For instance, the energy density of LIBs in EVs needs to be 2–5 times higher than the existing LIB technology (ca. 200 W h kg−1).6 At present, graphite is the most frequently used anode material in LIBs. However, its specific capacity (372 mA h g−1) is comparatively low to meet the increasing demand in high energy density.7 Therefore, alternative anode materials with various compositions and architectures are extensively explored in order to achieve excellent specific capacity, rate capability and cycling stability.8–11Recently, transition metal oxides (TMOs) have received much attention as promising anode materials for next generation LIBs, due to their high theoretical capacities (e.g. Co3O4: 890 mA h g−1, CoO: 715 mA h g−1, NiO: 718 mA h g−1).6,12,13 Particularly, Co3O4 has aroused considerable interest as its theoretical specific capacity is around 2–3 times higher than graphite. Unfortunately, Co3O4 suffers from poor cycling stability and poor rate capability in repeated charge–discharge cycles, due to huge volume expansion (about 100%) and low electrical conductivity.14,15 The rational design of nanostructures was proven to be one of the effective strategies to mitigate the problems arising from large volume variation and poor electron transport.16,17 So far, nanostructured Co3O4 anode materials with different morphologies have been examined, such as zero-dimensional nanostructures (e.g. nanoparticles),18 one-dimensional nanostructures (e.g. nanorods, nanotubes),19,20 two-dimensional nanostructures (e.g. nanosheets)21–23 and three-dimensional (3D) nanostructures (e.g. flower-like, urchin-like and pompon-like spheres).24–26 Generally, the reversible and fast transport of Li+ between the anode and the cathode is one of the main concerns for high performance electrode materials. Evidently, a flower-like structure, endowed with a 3D architecture, micro-/nanoscale dimension and continuous networks, is advantageous for alleviating volume expansion and enhancing charge (Li+ and electrons) transport in electrochemical processes. For example, Chen et al. compared lithium storage properties of cobalt-based nanocubes, nanodiscs and nanoflowers and confirmed the superior electrochemical performances of nanoflowers delivering a reversible capacity of 649 mA h g−1 after 100 cycles.27 Also, many other anode materials with a flower-like structure have already demonstrated excellent lithium storage performance, such as NiO, TiO2, SnO2 and NiCo2O4.28–31
Typically, the pursuit of high performance electrode materials has triggered the research interest in exploring flower-like Co3O4. For example, Jadhav et al. reported the chemical co-precipitation synthesis of flower-like Co3O4 composed of many porous nanorods of several micrometers in length.32 Also, Han et al. reported the hydrothermal synthesis of Co3O4 microscale flowers with sodium dodecyl sulfate (SDS) as a soft-template. The interaction between Co2+ ions and the hydrophilic terminus of the SDS surfactant was proposed to explain the nucleation and growth of flower-like microstructures.33 In addition, flower-like Co3O4 microspheres (ca. 3–4 µm in diameter) with nanosheets as building units were fabricated via a solvothermal route in the presence of succinic acid.24 Unfortunately, for previous studies, flower-like Co3O4 spheres applied in lithium storage performance evaluation were usually at the microscale.24 Based on the directly proportional relationship between diffusion time (τ) and the second power of diffusion length (λ2), the microscale diffusion length of Li+ was definitely detrimental to lithium storage capability.17 In this regard, it is of great significance to develop Co3O4 materials with smaller dimensions for next generation LIBs, such as nanoflower architectures.
Herein, mesoporous Co3O4 with nanoflower morphology (Co3O4-NF) was successfully fabricated via a facile ammonia-assisted solvothermal strategy. Ammonia played a crucial role as a complexing reagent in controlling nanoscale or microscale dimensions of flower-like Co3O4. By suitably increasing the amount of ammonia solution added, the dimension of a flower-like structure was tailored from nanoscale to microscale (Co3O4-MFs). Material characterization indicated that Co3O4-NFs were endowed with intriguing features for achieving excellent lithium storage, such as multi-scale dimensions, a high specific surface area (104 m2 g−1), a mesoporous architecture (22.6 nm) and a large pore volume (0.587 cm3 g−1). When evaluated at a high current density of 500 mA g−1 for 100 cycles, the performance results demonstrated the superior lithium storage of Co3O4-NFs in comparison with Co3O4-MFs and Co3O4-NPs. The specific discharge capacity of Co3O4-NFs after 100 cycles was about 1323 mA h g−1 without noticeable variation. The excellent C-rate performances of Co3O4-NFs also confirmed the great potential application in high-performance LIBs.
Experimental section
Synthesis of flower-like Co3O4-NFs and Co3O4-MFs
Flower-like Co3O4 precursors were first synthesized from cobalt sulphate in the presence of ethylene glycol, ammonia and sodium hydroxide by the solvothermal approach. In a typical process, 1.4 g of CoSO4·7H2O (≥99.0%, Sigma-Aldrich) was first dissolved in 30 mL of ethylene glycol (99.8%, Sigma-Aldrich), followed by adding 4 mL of concentrated ammonia solution (25–28%, Acros Organics). Meanwhile, 0.4 g of NaOH (≥98%, Sigma-Aldrich) was dissolved in 30 mL of ethylene glycol by magnetic stirring. The above solution was homogeneously mixed in a 50 mL Teflon-lined stainless-steel autoclave, followed by solvothermal treatment at 120 °C for 12 hours. After natural cooling, the as-prepared precursors were collected by vacuum filtration and rinsed 3 times with absolute ethanol. Finally, the as-prepared Co3O4 precursors were heat treated at 450 °C for 2 hours to obtain nanoflower Co3O4 products, denoted as Co3O4-NFs or Co3O4-NF. For comparison, Co3O4 products with a microflower structure, abbreviated as Co3O4-MFs or Co3O4-MF, were also prepared by increasing the added ammonia solution to 8 mL in the above solvothermal process. The as-prepared Co3O4-NFs and Co3O4-MFs were directly applied in material characterization and electrochemical tests.Material characterization
Morphologies and microstructures of flower-like Co3O4 precursors and products were identified using a field emission scanning electron microscope (FE-SEM, Hitachi S4800) and a transmission electron microscope (TEM, FEI Tecnai G2 20 scanning). The crystal phases of flower-like Co3O4 products were characterized using an X-ray diffractometer (XRD, Bruker) equipped with a LynxEye detector and Cu Kα12 X-ray radiation. The specific surface area and pore size distribution of Co3O4 products were obtained using Micromeritics ASAP 2020 by the Brunauer–Emmet–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods. Thermal conversion from precursors to Co3O4 products was investigated by thermogravimetric analysis (TGA 1, Mettler Toledo, STARe System) and differential scanning calorimetry (DSC 1, Mettler Toledo, STARe System) under an oxygen atmosphere with a heating rate of 10 °C min−1. The oxidation states of elements in precursors and products were investigated by X-ray photoelectron spectroscopy (XPS, Model PHI5600). The binding energy value in XPS was referenced to the C 1s peak at 284.6 eV.Electrochemical tests
Electrochemical performances of the as-prepared Co3O4 anode materials (Co3O4-NFs and Co3O4-MFs) were evaluated in a 2025 type coin cell at room temperature. In a typical process, Co3O4 anodes were prepared by mixing 70 wt% Co3O4 active materials with a 15 wt% conductive agent (acetylene black, Super P®) and a 15 wt% binder (polyvinylidene fluoride, PVDF) in N-methyl-2-pyrrolidinone (NMP) solvent. The as-prepared slurry was uniformly coated on a copper foil by employing an automatic film applicator (AFA-II, Shanghai Pushen Chemical Machinery Co. Ltd, China), followed by vacuum drying at 120 °C for 12 hours. A compact disc cutter (MSK-T-10, MTI Corporation) was used to prepare a Co3O4 anode disc (diameter: 15 mm). The mass loading of active anode materials was about 1 mg cm−2. The electrolyte was composed of a mixture of 50 vol% ethylene carbon and 50 vol% diethyl carbonate in 1 mol L−1 LiPF6. With the Co3O4 disc as the working electrode, lithium foil (diameter: 15 mm) as the counter electrode, and a polypropylene microporous membrane (Celgard 2400, diameter: 18 mm) as the separator, coin cells were assembled using a hydraulic crimping machine (MSK-110, MIT Corporation) in a glove box (MB-10, MBRAUN). The concentrations of oxygen and water were controlled at <0.5 ppm. Electrochemical impedance spectroscopy (EIS) of Co3O4 was conducted on an electrochemical work station (Bio-Logic, DHS) by providing a sine wave with an amplitude of 5 mV in the frequency range of 200 kHz–0.01 Hz. Battery performances of Co3O4 anodes were evaluated using a battery testing system LAND CT2001A (Wuhan Jinnuo Electronics, Ltd, China) with a cut-off voltage range of 0.01 V to 3.0 V vs. Li+/Li. A constant current density of 500 mA g−1 was applied to battery cycling performance evaluation for 100 cycles. For comparison, commercial Co3O4 micro-/nanoparticles (99.5%, Aladdin) abbreviated as Co3O4-NPs or Co3O4-NP were also tested under the same conditions. In addition, the C-rate performance of Co3O4-NF anode materials was studied by charge–discharge cycles with current densities of 100, 200, 500, 1000, 2000 and 3000 mA g−1.Results and discussion
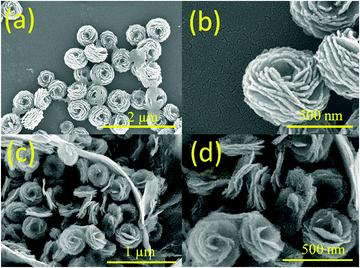
The morphologies and dimensions of the as-prepared cobalt-based precursors and products were investigated by FE-SEM. As shown in Fig. 1a and b, when 4 mL of ammonia solution were added for the solvothermal synthesis, nanoflower precursors of 580 ± 80 nm diameter could be clearly observed. The petals of each nanoflower were composed of thin nanosheets with around 10 nm thickness, and inter-connected with each other at the bottom of the nanosheets. When the precursors were calcined at 450 °C for 2 hours, nanoflower products (Co3O4-NFs) with 290 ± 25 nm diameter were still retained. The shrinkage of the diameter should be associated with the decomposition of precursors at high temperature. In Fig. 1c and d, nanosheets of around 10 nm thickness were clearly found in the 3D nanoflower products. This suggested that three-dimensional Co3O4 nanoflowers (Co3O4-NFs) were successfully prepared using the ammonia-assisted solvothermal method followed by heat treatment. With the well-connected structure, the multi-scale dimensions may lead to efficient electron transportation and lithium ion diffusion, indicating an improved charge transfer process in an electrochemical environment. Interestingly, when 8 mL of ammonia was added during the synthesis, flower-like precursors of larger dimensions were obtained. As illustrated in Fig. S1 (ESI†), the primary structure was nanosheets of a few nanometers and the secondary structure was spheres of a few micrometers (ca. 8 µm). Similarly, these flower-like precursors were converted into Co3O4 microflowers (Co3O4-MFs) by heat treatment at 450 °C. However, as seen in Fig. S2 (ESI†), further increasing ammonia amounts resulted in the formation of an irregular structure, probably due to dissolution of cobalt-based precursors under high pH conditions. As indicated in Fig. S3 (ESI†), by increasing the amount of ammonia from 2 mL to 12 mL, the pH values of the reactant solution before and after solvothermal treatment were gradually increased from 10.2–10.4 to 10.8–11.2. For the given ammonia amount, the decrease of the pH value was ascribed to the consumption of hydroxyl ions by the precipitation reaction. Note that the petal-like structure started to form when the amount of added ammonia solution was 2 mL. Based on the above concentration-dependent morphological study, ammonia was assumed to play a pivotal role as a complexing agent in controlling the dimensions of flower-like structures. Yang et al. previously reported the synthesis of flower-like cobalt-based precursors of 5–15 µm diameter by hydrothermal treatment of cobalt acetate in the presence of ethylene glycol.34 In this study, additional ammonia added during material synthesis could lead to a complexation reaction between cobalt ions and ammonia ions, resulting in the formation of stable [Co(NH3)6]2+.23 This species could effectively slow down material growth kinetics, thus reducing sphere dimensions from the microscale to nanoscale. Therefore, Co3O4-NFs and Co3O4-MFs could be easily tailored by varying the added amount of ammonia from 4 mL to 8 mL, respectively.The microstructure and crystallinity of Co3O4-NFs were investigated by TEM. Many Co3O4-NF spheres were found in Fig. 2a. As depicted in Fig. 2b, a typical Co3O4-NF sphere exhibited a diameter of about 260 nm, comparable with the FE-SEM result. Plenty of inter-connected nanoparticles of about 10 nm were found in the Co3O4-NFs. Uniform pores of around 20 nm in size were also observed. The mesoporous feature of Co3O4-NFs could effectively alleviate the problem of volume expansion during charge–discharge processes, meanwhile improving Li+ diffusion from the electrolyte to active sites for electrochemical reactions, resulting in superior cycling stability and rate capability.4 In addition, many inter-connected nanoparticles were present in Co3O4-NFs, which might be advantageous for effective electron transport. Note that Co3O4-NF products exhibited numerous nanoparticles by TEM characterization, which was different from the flower-like morphology by FE-SEM characterization. The reason for such a difference should be attributed to the different image formation principles and magnifications. The formation of TEM images was based on the transmitted electrons and could provide detailed two-dimensional microstructure information with a high magnification (195![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in Fig. 2b), while FE-SEM images were capable of giving three-dimensional surface morphology information but with a lower magnification (60
000 in Fig. 2b), while FE-SEM images were capable of giving three-dimensional surface morphology information but with a lower magnification (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in Fig. 1c) on the basis of the scattered electrons. The electron diffraction pattern in Fig. 2c implied that the as-prepared Co3O4-NFs exhibited a polycrystalline nature. The diffraction rings from inside to outside were indexed to the (111), (220), (311), (400), (422), (511) and (440) crystal planes of Co3O4. In Fig. 2d, the lattice spacing was measured to be 0.46 nm, corresponding to the (111) crystal plane of Co3O4. Therefore, TEM analysis further confirmed high crystallinity and mesoporous features of Co3O4-NFs.
000 in Fig. 1c) on the basis of the scattered electrons. The electron diffraction pattern in Fig. 2c implied that the as-prepared Co3O4-NFs exhibited a polycrystalline nature. The diffraction rings from inside to outside were indexed to the (111), (220), (311), (400), (422), (511) and (440) crystal planes of Co3O4. In Fig. 2d, the lattice spacing was measured to be 0.46 nm, corresponding to the (111) crystal plane of Co3O4. Therefore, TEM analysis further confirmed high crystallinity and mesoporous features of Co3O4-NFs.
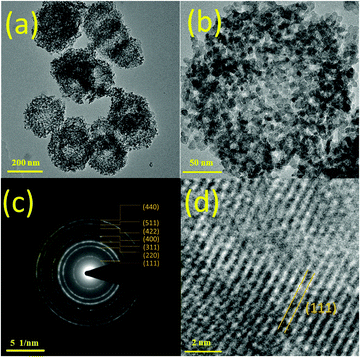 | ||
| Fig. 2 Typical TEM images of flower-like Co3O4-NFs and corresponding SAED pattern (a and b) TEM images of Co3O4-NFs; (c) SAED pattern; (d) HRTEM image. | ||
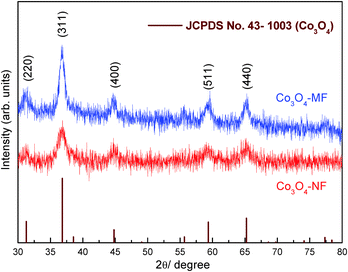
The crystalline structures of Co3O4 nanoflowers and microflowers were also characterized by XRD analysis. As shown in Fig. 3, XRD patterns of Co3O4-NFs and Co3O4-MFs were similar, as evidenced by the same diffraction peak positions. The observed 2θ peaks at 31.3°, 36.8°, 44.8°, 59.4° and 65.2° should be assigned to the (220), (311), (400), (511) and (440) crystal planes, respectively (JCPDS No. 43-1003). In addition, no significant impurities were detected in the final products, suggesting the complete conversion from the precursors to high purity Co3O4. Thus, the amounts of NH3 added had no influential effects on the crystal phase of the final products. The width of the Co3O4-NF (311) peak at half-height was much broader than that of Co3O4-MFs, suggesting the smaller dimension of crystallites in Co3O4-NFs. Based on the (311) diffraction peaks, the crystallite sizes of Co3O4-NFs and Co3O4-MFs were estimated by the Scherrer formula to be about 9 nm and 12 nm, respectively.35 In addition, the XRD results of cobalt-based flower-like precursors in Fig. S4 (ESI†) indicated a broad peak at about 35°, consistent with previous studies.36 Note that background noise in XRD patterns should be ascribed to the formation of fluoresced X-ray signals from transitional metal elements (e.g. Co) when Cu Kα12 was used for X-ray radiation. A similar noisy XRD background was also observed in previous studies on transition metal oxide materials.37,38
As shown in Fig. 4, N2 adsorption–desorption isotherms of Co3O4-NFs implied a typical IV isotherm with a hysteresis loop located in a relative pressure range (P/P0) of 0.5 to 0.97, indicating the existence of the mesoporous structure. In the insert of Fig. 4, the BJH pore size distribution curve of Co3O4-NFs implied that the average pore size was estimated to be 22.6 nm, comparable with TEM observation. However, no obvious pore size distribution was found in Co3O4-MFs, probably caused by the collapse of the sheet-like structure during heat treatment. As shown in Table S1 (ESI†), Co3O4-NFs showed a larger specific surface area (104 m2 g−1) and a larger pore volume (0.587 cm3 g−1), in comparison with Co3O4-MFs (83.6 m2 g−1 and 0.308 cm3 g−1). The large specific surface area of Co3O4-NFs should be ascribed to the multi-scale dimensions, including small nanoparticles (0D) of about 10 nm size, ultrathin nanosheets (2D) of about 10 nm thickness and nanoflowers (3D) of 290 ± 25 nm diameter. Not only the high specific surface area could provide more active sites for electrochemical reactions, but also enlarge the contact area between the anode and the electrolyte during lithiation–delithiation cycles.39 As a result, due to the presence of mesopores and their large pore volume, Co3O4-NFs were capable of accommodating drastic volume variation during electrochemical cycling.
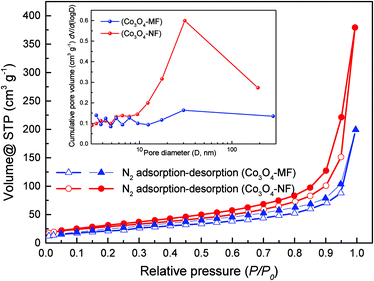 | ||
| Fig. 4 N2 adsorption–desorption isotherms of flower-like Co3O4-NFs and Co3O4-MFs (inset: pore size distributions of Co3O4-NFs and Co3O4-MFs). | ||
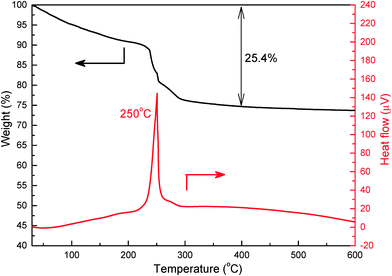
Thermal conversion from precursors to Co3O4 products was examined by TGA and DSC analysis. Representative TGA-DSC curves of flower-like precursors are displayed in Fig. 5. The initial weight loss of about 5% was probably attributed to the evaporation of water and ethylene glycol molecules trapped by the 3D flower-like precursors. With the increment in temperature, a significant weight loss of about 20% was observed at 100–300 °C. This could be ascribed to the decomposition of cobalt-based precursors, resulting in the formation of Co3O4 products and gaseous by-products (e.g. CO2 and H2O). No extra weight loss was found after 400 °C, indicating that 450 °C used in this study was sufficient to convert cobalt-based precursors into Co3O4. The sharp peak in the DSC curve suggested that the decomposition reaction occurred at about 250 °C. In addition, the TGA curve of Co3O4-NF products in Fig. S5 (ESI†) signified a small weight loss of around 2.5% upon heat treatment. This should be attributed to the removal of adsorbed water molecules from Co3O4-NFs, suggesting excellent thermal stability.
Element analysis and cobalt valences of Co3O4-NF products and precursors were analysed by XPS. The wide scan spectra in Fig. 6 suggested the existence of Co, O and C in both precursors and products. For Co3O4-NF products, the Co 2p3/2 peak could be split into two peaks at 781.2 and 779.4 eV, indicating the existence of Co2+ and Co3+, respectively.23 For flower-like precursors, the major peak was located at 780.8 eV, accompanied by a satellite peak of 785.8 eV, suggesting Co2+ in the precursors. It should be noted that no nitrogen elements was detected in these two samples, implying no chemical interactions between ammonia molecules and precursors (Fig. S6, ESI†). These results confirmed the formation of high purity Co3O4 materials using the ammonia-assisted solvothermal method followed by heat treatment.
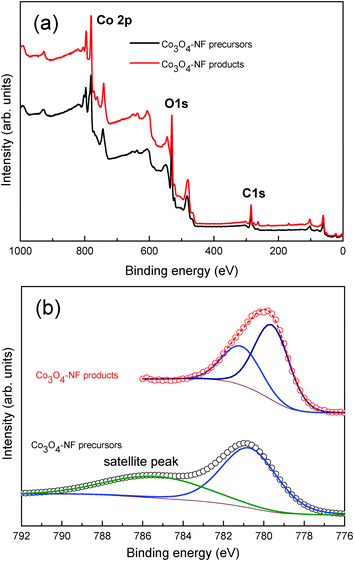 | ||
| Fig. 6 XPS analysis of flower-like precursors and Co3O4 nanoflowers (Co3O4-NFs) (a) wide scan; (b) high resolution Co 2p spectra. | ||
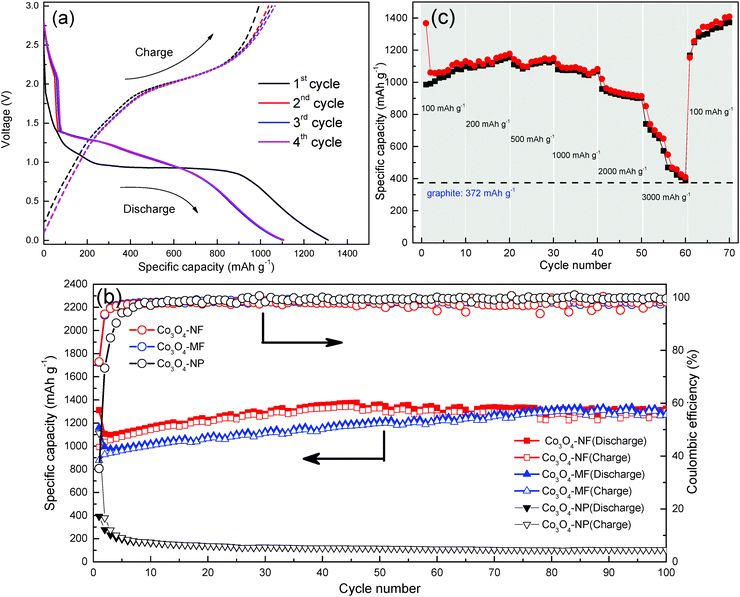
Electrochemical behaviours of Co3O4-NFs were investigated by charge–discharge profiles, as shown in Fig. 7a. The first four cycles were tested at a current density of 500 mA g−1 in a voltage range of 0.01–3.0 V (vs. Li+/Li). In this study, discharging and charging processes mean lithiation and delithiation, respectively. For the 1st discharge process, the voltage rapidly declined from the open-circuit voltage to approximately 1.0 V and a long plateau remained at around 0.9–1.0 V. The long plateau was mainly attributed to the redox conversion reaction involving Li and Co3O4. Co3O4 underwent the reduction process, which was being converted to an intermediate phase CoO, and finally metallic Co during the lithium insertion process. In the meantime, Li2O was formed by the oxidation process. The discharge curve further dropped to a cut-off voltage of 0.01 V. The sloping feature should be attributed to the formation of polymeric layers (e.g. solid electrolyte interphase, SEI) on Co3O4 active materials. In the reverse process, a charge plateau located at about 2.0 V should be ascribed to the reversible oxidation of metallic Co into Co3O4, accompanied by the release of Li+. The overall electrochemical reactions involved could be summarized into Co3O4 + 8Li+ + 8e− ↔ 4Li2O + 3Co.40,41 The initial discharge capacity and charge capacity were determined to be 1311.6 and 992.3 mA h g−1, respectively. For the 2nd, 3rd and 4th cycles, all of these cycles revealed similar profiles with a gradual drop. Their charge capacities were about 1036.5, 1051.9 and 1066.6 mA h g−1 respectively, while all of their discharge capacities were almost identical at around 1100 mA h g−1. The overlapping of charge–discharge profile suggested the identical electrochemical reaction pathways in subsequent cycles, indicating that stable SEI films were formed in the first charge–discharge cycle. It should be mentioned that the first cycle Coulombic efficiency of Co3O4-NFs was 75.6%, comparable or higher than previous studies on nanostructured Co3O4.42–45 For example, Li et al. reported mesoporous Co3O4 derived from a cobalt-based metal organic framework (MOF) exhibited a low Coulombic efficiency of 68%.42 Du et al. reported porous Co3O4 nanotubes templated from carbon nanotubes showed an initial Coulombic efficiency of about 70%.43 In addition, Fu et al. reported that lemongrass-like Co3O4 delivered a Coulombic efficiency of 73.2%.44 The capacity loss might be ascribed to the irreversible formation of lithium-containing SEI films (e.g. ROLi and ROCO2Li, R = alkyl group) in the first cycle.46 In addition, the obtained specific charge and discharge capacities in this study were much higher than the theoretical value of 890 mA h g−1. In fact, this phenomenon was common for cobalt-based and transition metal-based materials with a mesoporous structure.47–50 The extra capacities were probably attributed to the reversible formation of an electrochemically active gel-like film or interfacial lithium storage.51,52
As illustrated in Fig. 7b, lithium storage performances of different Co3O4 materials (Co3O4-NFs, Co3O4-MFs and Co3O4-NPs) were compared at a current density of 500 mA g−1 for 100 cycles. For Co3O4-NFs, both specific discharge and charge capacities were slightly increased for 40 cycles and then stabilized at about 1355 mA h g−1. After 100 cycles, the specific discharge capacity was approximately 1323 mA h g−1 and capacity retention was nearly 100%. For the cycling performance of Co3O4-MFs, the first specific discharge and charge capacities were 1160.9 and 876.6 mA h g−1 respectively, equivalent to a similar Coulombic efficiency of 75.5%. In the course of the 100 cycles, the charge and discharge capacities of Co3O4-MFs were gradually increased and after 100 cycles the final discharge was 1281 mA h g−1 with no obvious decay. The gradual increase of capacity for Co3O4-MFs was possibly due to the microscale dimension. In the initial charge–discharge cycles, partial lithiation and delithiation of Co3O4-MFs with a microscale dimension was possible at a high current density of 500 mA g−1, owing to the limited diffusion time for both lithium ions and electrons. With the proceeding of charge–discharge cycles, the activation of pores by repeated volume variation enabled the penetration of electrolytes into more active electrochemical sites, thus leading to the gradual increase of specific capacity.49 Actually, the gradual increase of specific capacity at the beginning of the charge–discharge cycles is a common phenomenon for Co3O4-based anode materials.27,49 In contrast, commercial Co3O4-NPs showed worse cycling performance in lithium storage. For the first cycle, the charge and discharge capacities were about 393 and 1112 mA h g−1, respectively. As indicated by the low Coulombic efficiency (ca. 35%), Co3O4-NPs exhibited serious irreversible electrochemical reactions in the first cycles, probably due to the significant formation of thick and irreversible SEI films. After 100 cycles, the specific discharge capacity was significantly decreased to about 107 mA h g−1. The typical FE-SEM image of commercial Co3O4 suggested the coexistence of nanoparticles of about 100 nm and microparticles of 1–2 µm in size (Fig. S7, ESI†). The apparent capacity fading of Co3O4-NPs was probably ascribed to the serious side reactions and agglomeration of Co3O4 micro-/nanoparticles in charge–discharge cycles.53 Wang et al. also reported that the specific capacity of spherical Co3O4 nanoparticles was severely declined from about 700 to 450 mA h g−1 after 50 cycles when tested at 100 mA g−1.54 The above results clearly demonstrated that Co3O4-NFs endowed with a unique structure and dimension were promising lithium storage materials for lithium ion batteries, in comparison with Co3O4-MFs and Co3O4-NPs. As shown in Table S2 (ESI†), from the view of capacity retention and discharge capacity, cycling performance of Co3O4-NFs was much better than most of the previous studies. For example, Co3O4 octahedra synthesized by Gao et al. delivered a capacity retention of 62.5% after 60 cycles at 500 mA g−1 and ordered mesoporous frameworks prepared by Fan et al. showed a capacity retention of 72.6% after 50 cycles at 890 mA g−1.55,56 In addition, after the cycling test, the specific discharge capacity of Co3O4-NFs was comparable or even higher than other nanostructures, such as nanospheres (820 mA h g−1@100 cycles) and snowflake-shaped Co3O4 (1044 mA h g−1@100 cycles).57 It should be mentioned that the pore size distribution of Co3O4-NFs (22.6 nm) was relatively larger than most of the previous studies (Table S2, ESI†). Too small pore size might be detrimental to fast electrolyte penetration and ionic diffusion.23,58 Note that Co3O4-NF materials demonstrated a robust structure after the charge–discharge of 100 cycles, as indicated in Fig. S8 (ESI†). This suggested that the mesoporous nature and large pore volume (0.587 cm3 g−1) of Co3O4-NFs could effectively alleviate the volume variation during lithiation/delithiation cycles, thereby resulting in excellent structural integrity.
In order to verify the excellent lithium storage capability of Co3O4-NFs at high current densities, the C-rate performance of Co3O4-NFs is shown in Fig. 7c. The average discharge capacity of Co3O4-NFs at a current density of 100, 200, 500 and 1000 mA g−1 was around 1084, 1140, 1122 and 1081 mA h g−1, respectively. It was clear that Co3O4-NFs maintained a stable and high specific capacity at current densities ranging from 100 to 1000 mA g−1. However, when the current density was further increased to 2000 and 3000 mA g−1, the average discharge capacity of Co3O4-NFs decreased to 925.9 and 570.8 mA h g−1, respectively. Although lithium storage performance was slightly decreased at high current densities, the achieved specific capacity was still much higher than the theoretical capacity of the existing graphite materials. Again, when Co3O4-NFs were tested at a current density of 100 mA g−1, an average discharge capacity of 1360 mA h g−1 was obtained, slightly higher than the previous cycles. The improved reversibility of Co3O4-NFs may be attributed to the activation of mesopores and nanostructured building units. By contrast, Co3O4-NPs exhibited poor rate capability in the range of 100 mA h g−1 to 3000 mA h g−1. As shown in Fig. S9 (ESI†), when current densities were 200, 500, 1000, 2000 and 3000 mA g−1, the specific discharge capacities were about 174, 127, 104, 85 and 74 mA h g−1, respectively. The lithium storage capacity of Co3O4-NPs even at a low current density (e.g. 200 mA g−1) was still lower than that of graphite. Previous work suggested that lithium storage capability at a high current density was determined by transport behaviours of both lithium ions and electrons.23 Although Co3O4-NPs containing small dimensions (nanoparticles of about 100 nm in size) could shorten the lithium ion diffusion length, electron transport between individual nanoparticles was previously suggested to follow a random walk in electrochemical processes, thus becoming the rate-determining step.22 Instead, owing to many inter-connected nanoparticles (about 10 nm) and a 3D flower-like structure, Co3O4-NFs possessed not only a short lithium ion diffusion length but also improved 3D inter-connected electron transport networks. Thus, the C-rate performances of the Co3O4 electrode could be manipulated by material morphology and structure dimension, which had strong influences on lithium diffusion and electron transport networks.
In order to understand the superior lithium storage performance of Co3O4-NFs, EIS was conducted on fresh coin cells assembled with different Co3O4 architectures, including nanoflowers, microflowers and micro-/nanoparticles. As indicated in Fig. 8, Nyquist plots of Co3O4-NFs, Co3O4-MFs and Co3O4-NPs showed a depressed semicircle in the high-middle frequency region and an inclined line in the low frequency region, consistent with previous studies on Co3O4-based anode materials.39,59 The equivalent circuit for fitting the measured impedance data is also given in the inset of Fig. 8. The electrolyte resistances (R1) of Co3O4-NFs, Co3O4-MFs and Co3O4-NPs calculated from the intersection point at a high frequency real axis were about 4, 10 and 4 ohm, respectively. In addition, the charge transfer resistances (R2) of Co3O4-NFs, Co3O4-MFs and Co3O4-NPs determined by the diameters of the semicircles were about 35, 89 and 141 ohm, respectively. Apparently, Co3O4-NFs exhibited the smallest charge transfer resistance, indicating fast transportation of lithium ions and electrons in an electrochemical environment. The inclined line in the lower frequency region was characteristic of Warburg impedance, which should be relevant to lithium ion diffusion. The improvement of charge transfer and lithium ion diffusion should be probably attributed to the intriguing structural characteristics of Co3O4-NFs.
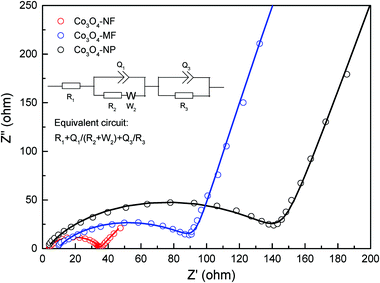 | ||
| Fig. 8 Nyquist plots of Co3O4-NFs, Co3O4-MFs and Co3O4-NPs (inset: the equivalent circuit adopted in the fitting of all the measured impedance data; solid line: fitting data). | ||
In conclusion, Co3O4-NFs endowed with a nanoflower structure manifested better lithium storage performances, compared to Co3O4-MFs with a microflower structure and Co3O4-NPs with a micro-/nanoparticle structure. The remarkable lithium storage performances should be related to the unique structural characteristics, which contributed to the enhanced transportation of electrons and lithium ions during charge–discharge cycles. To be specific, first, multi-scale dimensions of Co3O4-NFs, including 0D nanoparticles, 2D nanosheets and 3D nanoflowers could greatly shorten the lithium ion diffusion length and improve electron transport networks. Second, the large specific surface area could provide more active sites and enlarge the contact area between anode materials and electrolyte for electrochemical reactions. And third, the mesoporous nature and the large pore volume could ensure excellent electrode integrity by providing sufficient space to alleviate volume variations in lithiation–delithiation cycles. These intriguing merits of Co3O4-NFs guaranteed the superior lithium storage capability, suggesting that the rational structure design was a promising solution to address the challenges of high-capacity anode materials.
Conclusions
In summary, Co3O4 nanoflowers (Co3O4-NFs) with multi-scale dimensions and a mesoporous structure were successfully synthesized using a facile ammonia-assisted solvothermal method, followed by heat treatment at 450 °C for 2 h. By increasing the amount of added ammonia solution in solvothermal synthesis, the nanoflower structure was correspondingly tailored into microflowers (Co3O4-MFs). When investigated as anode materials at a current density of 500 mA g−1 for 100 cycles, Co3O4-NFs delivered a reversible specific capacity of 1323 mA h g−1, much better than Co3O4-MFs (1281 mA h g−1) and Co3O4-NPs (107 mA h g−1). In addition, Co3O4-NFs manifested better C-rate performances at high current densities. Specifically, when current densities used in charge–discharge cycles were further increased to 1000, 2000 and 3000 mA g−1, the average reversible specific capacities of Co3O4-NFs were around 1081, 925.9 and 570.8 mA h g−1, respectively. The enhanced lithium storage capability should be attributed to the unique characteristics of Co3O4-NFs, including multi-scale dimensions, a large specific surface area, mesoporous nature and a large pore volume. Overall, this work demonstrated great potential of Co3O4 nanoflowers as promising anode materials for applications in next generation lithium ion batteries.Acknowledgements
The research work presented in this paper was financially supported by Technological and Higher Education Institute of Hong Kong Seed Grant Scheme (No. 1415103 and 14151112).Notes and references
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- B. Dunn, H. Kamath and J.-M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- J. B. Goodenough and K.-S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS PubMed.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587–603 CrossRef CAS.
- S. Goriparti, E. Miele, F. De Angelis, E. Di Fabrizio, R. Proietti Zaccaria and C. Capiglia, J. Power Sources, 2014, 257, 421–443 CrossRef CAS.
- A. Manthiram, J. Phys. Chem. Lett., 2011, 2, 176–184 CrossRef CAS.
- H. B. Wu, J. S. Chen, H. H. Hng and X. Wen Lou, Nanoscale, 2012, 4, 2526–2542 RSC.
- P. Roy and S. K. Srivastava, J. Mater. Chem. A, 2015, 3, 2454–2484 CAS.
- J. Liu and X.-W. Liu, Adv. Mater., 2012, 24, 4097–4111 CrossRef CAS PubMed.
- L. Lai, J. Zhu, Z. Li, D. Y. W. Yu, S. Jiang, X. Cai, Q. Yan, Y. M. Lam, Z. Shen and J. Lin, Nano Energy, 2014, 3, 134–143 CrossRef CAS.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS PubMed.
- L. Liu, Y. Guo, Y. Wang, X. Yang, S. Wang and H. Guo, Electrochim. Acta, 2013, 114, 42–47 CrossRef CAS.
- J. Guo, L. Chen, X. Zhang, B. Jiang and L. Ma, Electrochim. Acta, 2014, 129, 410–415 CrossRef CAS.
- Y. M. Chen, L. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2016, 55, 5990–5993 CrossRef CAS PubMed.
- Y. Wang, H. Xia, L. Lu and J. Lin, ACS Nano, 2010, 4, 1425–1432 CrossRef CAS PubMed.
- Y. Tang, Y. Zhang, W. Li, B. Ma and X. Chen, Chem. Soc. Rev., 2015, 44, 5926–5940 RSC.
- L. Shen and C. Wang, Electrochim. Acta, 2014, 133, 16–22 CrossRef CAS.
- R. Xu, J. Wang, Q. Li, G. Sun, E. Wang, S. Li, J. Gu and M. Ju, J. Solid State Chem., 2009, 182, 3177–3182 CrossRef CAS.
- X. W. Lou, D. Deng, J. Y. Lee, J. Feng and L. A. Archer, Adv. Mater., 2008, 20, 258–262 CrossRef CAS.
- F. Zhan, B. Geng and Y. Guo, Chem. – Eur. J., 2009, 15, 6169–6174 CrossRef CAS PubMed.
- B. Wang, X.-Y. Lu, Y. Tang and W. Ben, ChemElectroChem, 2016, 3, 55–65 CrossRef CAS.
- B. Wang, X.-Y. Lu and Y. Tang, J. Mater. Chem. A, 2015, 3, 9689–9699 CAS.
- J. Zheng, J. Liu, D. Lv, Q. Kuang, Z. Jiang, Z. Xie, R. Huang and L. Zheng, J. Solid State Chem., 2010, 183, 600–605 CrossRef CAS.
- X. Rui, H. Tan, D. Sim, W. Liu, C. Xu, H. H. Hng, R. Yazami, T. M. Lim and Q. Yan, J. Power Sources, 2013, 222, 97–102 CrossRef CAS.
- W. Hao, S. Chen, Y. Cai, L. Zhang, Z. Li and S. Zhang, J. Mater. Chem. A, 2014, 2, 13801–13804 CAS.
- J. S. Chen, T. Zhu, Q. H. Hu, J. Gao, F. Su, S. Z. Qiao and X. W. Lou, ACS Appl. Mater. Interfaces, 2010, 2, 3628–3635 CAS.
- G. H. Yue, Y. C. Zhao, C. G. Wang, X. X. Zhang, X. Q. Zhang and Q. S. Xie, Electrochim. Acta, 2015, 152, 315–322 CrossRef CAS.
- Z. Zhang, Z. Zhou, S. Nie, H. Wang, H. Peng, G. Li and K. Chen, J. Power Sources, 2014, 267, 388–393 CrossRef CAS.
- H. Wang, Q. Liang, W. Wang, Y. An, J. Li and L. Guo, Cryst. Growth Des., 2011, 11, 2942–2947 CAS.
- L. Li, Y. Cheah, Y. Ko, P. Teh, G. Wee, C. Wong, S. Peng and M. Srinivasan, J. Mater. Chem. A, 2013, 1, 10935–10941 CAS.
- H. S. Jadhav, A. K. Rai, J. Y. Lee, J. Kim and C.-J. Park, Electrochim. Acta, 2014, 146, 270–277 CrossRef CAS.
- L. Han, Y. Cai, P. Tang and L. Zhang, Mater. Today, 2015, 18, 410–411 CrossRef.
- L.-X. Yang, Y.-J. Zhu, L. Li, L. Zhang, H. Tong, W.-W. Wang, G.-F. Cheng and J.-F. Zhu, Eur. J. Inorg. Chem., 2006, 4787–4792 CrossRef CAS.
- B. Wang, X.-Y. Lu, L. K. Yu, J. Xuan, M. K. H. Leung and H. Guo, CrystEngComm, 2014, 16, 10046–10055 RSC.
- J. Jiang, W. Shi, S. Song, Q. Hao, W. Fan, X. Xia, X. Zhang, Q. Wang, C. Liu and D. Yan, J. Power Sources, 2014, 248, 1281–1289 CrossRef CAS.
- Y. Wang, A. Pan, Q. Zhu, Z. Nie, Y. Zhang, Y. Tang, S. Liang and G. Cao, J. Power Sources, 2014, 272, 107–112 CrossRef CAS.
- J. Xu, L. He, W. Xu, H. Tang, H. Liu, T. Han, C. Zhang and Y. Zhang, Electrochim. Acta, 2014, 145, 185–192 CrossRef CAS.
- Y. Xiao, C. Hu and M. Cao, J. Power Sources, 2014, 247, 49–56 CrossRef CAS.
- H. Song, L. Shen and C. Wang, J. Mater. Chem. A, 2014, 2, 20597–20604 CAS.
- H. Sun, X. Sun, T. Hu, M. Yu, F. Lu and J. Lian, J. Phys. Chem. C, 2014, 118, 2263–2272 CAS.
- C. Li, T. Chen, W. Xu, X. Lou, L. Pan, Q. Chen and B. Hu, J. Mater. Chem. A, 2015, 3, 5585–5591 CAS.
- N. Du, H. Zhang, B. D. Chen, J. B. Wu, X. Y. Ma, Z. H. Liu, Y. Q. Zhang, D. R. Yang, X. H. Huang and J. P. Tu, Adv. Mater., 2007, 19, 4505–4509 CrossRef CAS.
- Y. Fu, X. Li, X. Sun, X. Wang, D. Liu and D. He, J. Mater. Chem., 2012, 22, 17429–17431 RSC.
- H. Chen, Q. Zhang, J. Wang, D. Xu, X. Li, Y. Yang and K. Zhang, J. Mater. Chem. A, 2014, 2, 8483–8490 CAS.
- S. J. An, J. Li, C. Daniel, D. Mohanty, S. Nagpure and D. L. Wood Iii, Carbon, 2016, 105, 52–76 CrossRef CAS.
- H. Huang, W. Zhu, X. Tao, Y. Xia, Z. Yu, J. Fang, Y. Gan and W. Zhang, ACS Appl. Mater. Interfaces, 2012, 4, 5974–5980 CAS.
- K. M. Shaju, F. Jiao, A. Debart and P. G. Bruce, Phys. Chem. Chem. Phys., 2007, 9, 1837–1842 RSC.
- B. Wang, Y. Tang, X.-Y. Lu, S. L. Fung, K. Y. Wong, W. K. Au and P. Wu, Phys. Chem. Chem. Phys., 2016, 18, 4911–4923 RSC.
- J. S. Chen, Y. Zhang and X. W. Lou, ACS Appl. Mater. Interfaces, 2011, 3, 3276–3279 CAS.
- M. Y. Son, J. H. Kim and Y. C. Kang, Electrochim. Acta, 2014, 116, 44–50 CrossRef CAS.
- J. Shao, Z. Wan, H. Liu, H. Zheng, T. Gao, M. Shen, Q. Qu and H. Zheng, J. Mater. Chem. A, 2014, 2, 12194–12200 CAS.
- W. W. Lee and J.-M. Lee, J. Mater. Chem. A, 2014, 2, 1589–1626 CAS.
- D. Wang, Y. Yu, H. He, J. Wang, W. Zhou and H. D. Abruña, ACS Nano, 2015, 9, 1775–1781 CrossRef CAS PubMed.
- J. Guo, L. Chen, X. Zhang, B. Jiang and L. Ma, Electrochim. Acta, 2014, 129, 410–415 CrossRef CAS.
- S. Fan, X. Liu, Y. Li, E. Yan, C. Wang, J. Liu and Y. Zhang, Mater. Lett., 2013, 91, 291–293 CrossRef CAS.
- D. Ge, H. Geng, J. Wang, J. Zheng, Y. Pan, X. Cao and H. Gu, Nanoscale, 2014, 6, 9689–9694 RSC.
- D. Li, L.-X. Ding, H. Chen, S. Wang, Z. Li, M. Zhu and H. Wang, J. Mater. Chem. A, 2014, 2, 16617–16622 CAS.
- Y. Wang, B. Wang, F. Xiao, Z. Huang, Y. Wang, C. Richardson, Z. Chen, L. Jiao and H. Yuan, J. Power Sources, 2015, 298, 203–208 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: FE-SEM images, pH values as a function of added ammonia, XRD patterns of cobalt-based precursors, the TGA curve of Co3O4-NFs, XPS high resolution N 1s spectra, C-rate performance of Co3O4-NPs, specific surface areas, pore properties and performance comparison table. See DOI: 10.1039/c6qm00114a |
| This journal is © the Partner Organisations 2017 |