Boosting the ultrastable Li storage performance in electron-sponge-like polyoxovanadates by constructing inorganic 3D structures†
Shanshan
Lu‡
a,
Yang
Lv‡
b,
Wenqing
Ma
a,
Xiaofeng
Lei
a,
Ruie
Zhang
a,
Hong
Liu
 *b and
Xizheng
Liu
*b and
Xizheng
Liu
 *a
*a
aTianjin Key Laboratory of Advanced Functional Porous Materials, School of Materials Science and Engineering, Tianjin University of technology, Tianjin 300350, P.R. China. E-mail: xzliu@tjut.edu.cn
bCollege of Pharmacy, Jiamusi University, Jiamusi 154007, P.R. China. E-mail: hliu@jmsu.edu.cn
First published on 13th October 2017
Abstract
Polyoxometalates act as an electron sponge, processing multielectron redox reactions and acting as a fast ionic conductor. They show great potential as promising electrode materials for next-generation lithium ion batteries (LIBs). However, there are still some fundamental issues which should be solved before their application can be realized, such as determining the stable structural feature with reversible Li+ ion insertion/desertion. In this work, polyoxovanadates (POVs) based materials of K4Na2V10O28·nH2O (KNaV10) and Mg2(NH4)2V10O28·nH2O (MgV10) have been prepared and used as the electrode material for a Li+ ion reservoir. The 10-core polyoxovanadate is demonstrated as a anionic building block and the 3D extended structure has been smartly tuned by counter cations. For MgV10, a 1D tunnel with an approximate size of 3 Å × 10 Å was formed along the a axis by Mg2+ ions and [V10O28]6− polyanions. The MgV10 shows a higher capacity, cycling stability, and rate performance than that of KNaV10 without tunnels. The capacity of MgV10 is about 160 mA h g−1 at a high discharge rate of 250 mA g−1, while it is only 118 mA h g−1 for KNaV10. Even after 60 discharge/charge cycles at 50 mA g−1, it displayed a capacity of 180 mA h g−1. The 1D tunnel in MgV10 facilitates the Li+ ion transport and provides spatial Li storage sites, which promotes the electrochemical performance in LIBs. Moreover, the Mg2+ ions remained stable during battery cycling and promoted the 3D structure stability. This work demonstrates promising guidelines for the structural design of POVs based materials for Li storage.
Introduction
The increasing demand for more advanced consumer electronics, electric vehicles, and stationary facilities calls for next generation rechargeable batteries with higher specific and volumetric capacities than the current state-of-the-art lithium ion batteries (LIBs).1–3 To date, the distorted rock-salt α-NaFeO2, spinel, and olivine structured bulk transition metal oxides are still the mainstream of the current cathode materials.4–6 However, these compounds are still challenged by lower capacity and limited Li+ ion transport kinetics, resulting in a relatively low power density output which limits their further applications.7–9 In this regard, novel electrode materials that process multi-electron redox reactions and enable more rapid Li+ ion transportation are needed to satisfy the increasing demand for high power and energy density. Among the potential candidates, the highly redox-active molecular metal oxides, widely known as polyoxometalates (POMs),10 have been investigated as promising electrode materials for their distinguished physical/chemical properties such as electronic versatility, versatile structures, and unique molecular structures etc.11–13 Great progress has been achieved. For instance, Song et al. reported the periodic patterning redox-active POMs on individual carbon nanotubes (CNTs), where the chemical activity and battery performance can be tuned at the molecular level with a diverse range of element doping.14 Ma et al. developed a bottom-up strategy to fabricate hierarchically sandwiched frameworks composed of manganese oxide nanosheets and various guest metal cations, which showed good electrochemical performance for both Li and Na storage.15 Recently, Lan and Sha et al. fabricated a series of Keggin type POMs/metal organic framework hybrids and explored these as anodes for LIBs. The corresponding electrochemical performance was better than that with simple counter cations.16 Most of the works have concentrated on composite design and fabrication to improve their conductivity and stability.17–20 There is still lack of fundamental understanding related to the stability of polyanions, as well as the 3D structures upon discharge/charge cycles. To further expand their application and obtain a more stable high performance from POMs based electrode materials, investigation of the structural effects and structural design guidelines are urgently needed.Herein, we demonstrate 3D polyoxovanadates (POVs) with multielectron redox properties as high performance electrode materials. The extended 3D structures have been designed and smartly tuned by optimizing the counter cations. The [V10O28]6− functions as an “electron sponge”, which can theoretically reversibly adopt 10 electrons between [V10O28]6− and [V10O28]16− during charging/discharging. Our results revealed that the electrochemical properties such as capacities and cyclability of the electrode materials are influenced by the counter-cations (K+ or Mg2+) directed structural effects, suggesting the crucial role of these guest cations. The fundamental findings presented in this work provide new guidelines for the optimization of the host materials with fast Li+ ion transportation and a robust structure for next generation LIBs.
Experimental
Synthesis of KNaV10
KNaV10 was synthesized according to the literature method.21 V2O5 1 g (5 mmol) was dissolved in 50 mL of 0.5 M CH3COOH/CH3COONa buffer solution (pH = 4.8) with stirring. The turbid solution was heated at 80 °C for 1 h. Then the solution was filtered off and KCl (3.5 g) was added to the solution and the mixture was stirred for another 15 min. The orange single crystals suitable for crystallography were obtained from the solution and the crystal samples air-dried at room temperature for further characterization.Synthesis of MgV10
MgV10 was synthesized by the following procedure: NH4VO3 2.1 g (17.95 mmol) was dissolved in deionized water (50 mL) and heated at 100 °C for several minutes until the solution became yellow and transparent. After cooling down to room temperature, a Mg(NO3)2 solution (1.4 g, 5.5 mmol Mg(NO3)2, 10 mL H2O) was added to the above solution under stirring. The pH of the resulting yellow clear solution was adjusted to 4.4 and evaporated slowly at room temperature. The orange crystals were obtained and air-dried at room temperature. Both of the structures were determined by single crystal X-ray diffraction analysis.Materials characterization
The crystal structure of POVs was determined by a Bruker SMART CCD APEX II diffractometer with a graphite monochromated Mo K source of (λ = 0.71073 Å) at 293 K. All data were corrected for Lorentz polarization and absorption effects. X-ray diffraction (XRD) patterns were obtained using a Bruker D8 Advance X-ray diffractometer employing Cu Kα radiation (λ = 0.15406 nm). The thermal stability was tested by thermogravimetric (TG) analysis (Netzsch DSC 214) under a nitrogen atmosphere at a heating rate of 10 °C min−1. The SHELXL2014 programs were used for structural determination and refinement.22 The structure was solved by the least-squares methods. Except for some disordered O atoms, all other non-hydrogen atoms were refined anisotropically by least-squares on F2 using Olex2.23 FTIR characterization was carried out on a BRUKER Vertex 70 spectrometer with a KBr pellet in the 4000–400 cm−1 region. Particle size and morphology were observed by a scanning electron microscopy (SEM, Quanta FEG 250, FEI) operating at 10 kV and a transmission electron microscope (TEM, Tecnai G2 Spirit TWIN, FEI) operating at 120 kV. The surface elemental information of the POMs were analysed by means of an X-ray photoelectron spectrometer (XPS, ESCALAB 250Xi, ThermoFisher Scientific), using monochromatized Al Kα X-rays as the excitation source and C 1s (284.60 eV) as the reference line.Electrochemical measurements
The electrochemical properties of POVs were tested with two-electrode 2032 coin-type cells using pure Li foil (Aldrich) as the counter electrodes. The cathode composition was POVs, acetylene black (AB) and polytetrafluroethylene preparation (60% dispersion in H2O, Sigma Aldrich Co.) at a weight ratio of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The mixture was then rolled onto the aluminium mesh (100 meshes) and dried at 60 °C under vacuum for 12 h. The electrolyte was LiPF6 (1 M) in a mixture of dimethyl carbonate and ethylene carbonate (1
10. The mixture was then rolled onto the aluminium mesh (100 meshes) and dried at 60 °C under vacuum for 12 h. The electrolyte was LiPF6 (1 M) in a mixture of dimethyl carbonate and ethylene carbonate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, by volume). A microporous Clegard 2400 was used as a separator. The cells were assembled in an Ar-filled glove-box with less than 0.1 ppm of both oxygen and moisture. Galvanostatic discharge/charge measurements were performed in the potential window from 1.0 to 3.8 V versus Li+/Li on an Arbin battery test system (BT2043). Cyclic voltammetry (CV) data were collected by using an electrochemical workstation (CHI 760E) in the potential window of 3.8 to 1.0 V at a scan rate of 0.1 mV s−1.
1, by volume). A microporous Clegard 2400 was used as a separator. The cells were assembled in an Ar-filled glove-box with less than 0.1 ppm of both oxygen and moisture. Galvanostatic discharge/charge measurements were performed in the potential window from 1.0 to 3.8 V versus Li+/Li on an Arbin battery test system (BT2043). Cyclic voltammetry (CV) data were collected by using an electrochemical workstation (CHI 760E) in the potential window of 3.8 to 1.0 V at a scan rate of 0.1 mV s−1.
Results and discussion
Single crystal X-ray diffraction analysis reveals that KNaV10 is composed of [V10O28]6− polyoxoanion, K+, Na+, and water molecules. The [V10O28]6− polyoxoanion is a 10-core polyoxovanadate anion, which is constructed by ten VO6 polyhedra that arrange each other by corner- or edge-sharing modes. The K+ and Na+ ions not only compensate the charge balance, but also coexist with structural water molecules to form a fully filled 3D framework, in which Na+ ions coordinate to the [V10O28]6− anion by Na–O bonds while K+ ions form the dimer units (Fig. S1†). The 3D packing diagram of KNaV10 indicates that there is not any porous channel formed in the solid state.For complex MgV10, when Mg2+ ions and NH4+ ions are used as counter cations in the synthetic design, it is different to the [V10O28]6− polyoxoanion in KNaV10 as the Mg2+ ions and solvent water molecules in MgV10 link the neighboring [V10O28]6− only by diverse hydrogen bonds to form the supermolecular structure.24 We performed TG analysis to analyze the water content in the prepared samples. As shown in the Fig. S2,† there are two weight loss processes corresponding to the water molecules. An early weight loss from 40 °C to 110 °C is associated with the loss of free water molecules. A consecutive steady weight loss from 110 °C to 250 °C corresponds to the loss of crystal water. This is due to the hydrogen bonds formed by the crystal water molecule and is similar to the previous reports.25 Furthermore, omitting the water molecules, an interesting 1D channel can be observed along the a axis with a size of ca. 3 Å × 10 Å (Fig. 1a). These 1D channels may facilitate the Li+ ions migration (Fig. 1b). As such, the syntheses of KNaV10 and MgV10 pave the way to the rational design of porous polyoxovanadate materials by selection of suitable cations. Both the experimental and simulated XRD patterns for the two materials are shown in the Fig. S3 and S4.† The main XRD peaks of the prepared bulk materials are consistent with the simulated data, which demonstrates the high purity of prepared compounds.
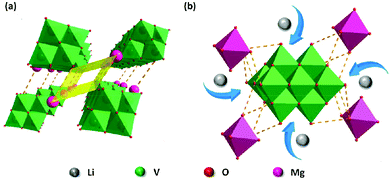 | ||
| Fig. 1 (a) The crystal structure of MgV10. Green: vanadium; red: oxygen; pink: magnesium. (b) Expected lithiation process of the MgV10 material. | ||
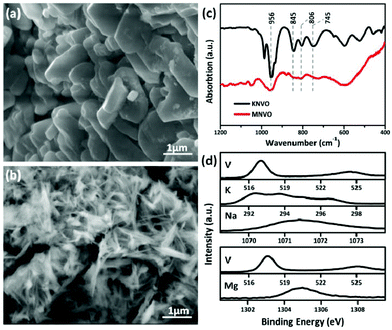
The morphology and structure of the POVs were studied by SEM, TEM, FTIR and XPS. As shown in Fig. 2a and b, the dehydrated KNaV10 clusters form irregular particles with a size around 1 μm, while the MgV10 demonstrates an interconnected, open and porous structure constructed by numerous nanosheets with a width of about 60 nm (Fig. S5†). The different morphology was associated with the counter cation directed crystallization process.26,27 The solubility product constant of K+ and Na+ surpasses that of Mg2+, and the crystallization speed of KNaV10 is slower than MgV10. Therefore, MgV10 exhibited a sheet like morphology while KNaV10 displays as particles with a scale of several hundred nm. As we know, the morphology can profoundly affect the electrochemical performance.28 FTIR analyses provided further structural information of the [V10O28]6−. As shown in Fig. 2c, the strong band located at ∼956 cm−1 could be ascribed to the stretching of the terminal V–O bonds. While the other three bands at around 745, 806, and 845 cm−1 possibly correspond to the bridging antisymmetric vibrations of V–O–V.21 The FTIR spectra of KNaV10 and MgV10 are not completely overlapped as observed in Fig. 2c, which suggests that the stretching of the polyanions bands have been deeply influenced by the counter-cations. The XPS of KNaV10 and MgV10 samples feature a couple of peaks located at 517.20 and 524.90 eV, which could be assigned to the V5+ species from the fitting of the V 2p standard spectra (Fig. 2d).29 The XPS peaks of Na 1s (1071.50 eV), K 2p (292.60 and 295.00 eV),15 and Mg 1s (1304.70 eV)30 confirm the successful incorporation of these guest cations into the [V10O28]6– frameworks. Based on these characterizations, the POVs samples of KNaV10 and MgV10 have been successfully synthesized as deigned.
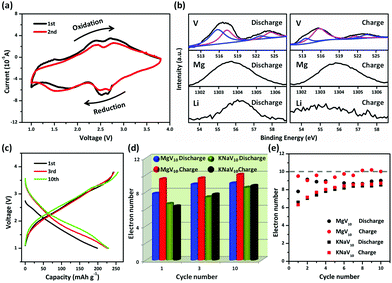
The reversible electrochemical performance of POVs has been carefully examined. The CV data for MgV10 reveals the redox properties of the POV during reversible Li+ ions insertion/desertion. As shown in Fig. 3a, two redox couples were observed which demonstrated a small voltage separation. The first two cycles of CV curves overlapped well, indicating functional stability as an electrode in LIBs and a higher stability of MgV10 than that of KNaV10 (Fig. S6†). The morphology of electrodes after discharging/charging shown in Fig. S7† also demonstrated that the MgV10 remained stable. Fig. 3b shows the ex situ XPS characterizations of the electrode at different discharge and charge stages. It can be seen that the POV after discharging showed complicated V 2p peaks (highlighted with blue and pink lines), which could be mainly assigned to the V4+ and V5+ species from the fitting of the V 2p standard spectra. The presence of the V5+ species in the discharging state might arise from the incomplete reduction in the POV framework.31 Whereas, after charging, the V5+ species32 increasing clearly and the appearance of the V4+ species can be attributed to the incomplete oxidation of the V centers, which corresponds to the battery performance. The reversible redox of the V center enables the Li storage in POVs based materials. The intensity of Mg2+ ions exhibits no obvious changes in different charging/discharging states. We performed FTIR to further characterize the structure and stability of POVs anions before and after cycles. As shown in Fig. S8 and S9,† the fingerprint spectrum between 800 and 1100 cm−1, which originated from the vibration of the polyanions, experienced no obvious changes. These results demonstrate that the POVs anions remained almost stable during cycles.
To further study their electrochemical performance, a galvanostatic discharge/charge was carried out to evaluate the Li storage performance. The MgV10 (KNaV10) shows an initial discharging capacity of 198.8 (152.6) mA h g−1 at a current density of 50 mA g−1 (Fig. 3c, Fig. S10†). Accordingly, the theoretical specific capacities33 of MgV10 and KNaV10 can be calculated as 257.25 and 231.08 mA h g−1, respectively. For the 10 electrons redox reactions,34 the migration of each Li+ ion contributes a capacity of around 25.7 (MgV10) and 23.1 (KNaV10) mA h g−1. Therefore, about eight (198.8/25.7)/seven (152.6/23.1) V5+ centers can be reduced if all of the V species are reduced to V4+ (Fig. 3d). Whereas, there are about ten (seven) V4+ centers that undergo electrochemical oxidization during the subsequent charging process for MgV10 (KNaV10). It can be expected, theoretically, that 10-electrons reactions can occur from the 10th charge process (the theoretical redox electrons are shown in Fig. 3e as dashed lines). With prolonged cycles, the MgV10 can reversible uptake more electrons than that of KNaV10, which reveals that the improved structural stability is brought by the stable Mg2+ ions providing support to POV frameworks.
The above discussion disclosed that the POVs underwent a dynamic activation process in the initial 10 cycles. To evaluate the electrochemical properties of the POVs as a cathode material, rate performance and cycling stability were performed. MgV10 shows a higher rate performance than KNaV10 when subjected to various current densities over the voltage range 1.0–3.8 V (Fig. 4a). There is no severe polarization when the MgV10 was subjected to a high charging–discharging current density. The capacity is around 160 mA h g−1 at a high discharge current density of 250 mA g−1, while it is only 118 mA h g−1 for KNaV10. This excellent rate performance could be attributed to the 3D interconnected, porous, and open structured feature (Fig. 2b, Fig. S11†).35 Cycle performance was further conducted at a current density of 50 mA g−1. As shown in Fig. 4b, the MgV10 exhibits a high discharge capacity of around 180 mA h g−1 up to the 60th cycles. In contrast, the specific capacities of KNaV10 significantly decreased after 10 cycles, and after 60 cycles, the capacity retention is just 63.9% of the 10th cycle (Fig. S10 and S12†). Such a prominent difference between MgV10 and KNaV10 highlights the effects of the structural stability, large accessible surface area, and more facile active sites in MgV10 that are favorable for fast Li+ ions diffusion and storage.
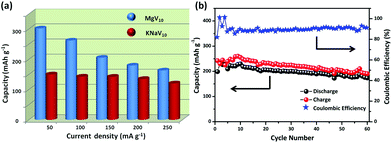 | ||
| Fig. 4 (a) Schematic illustration of the discharge capacity evolutions of MgV10 and KNaV10 at different current densities. (b) Cycling performance and coulombic efficiency of MgV10 at 50 mA g−1. | ||
Conclusions
In conclusion, we designed and fabricated POVs based electrode materials and systematically investigated the extended stable 3D structure feature for fast Li+ ion transportion and storage. The present results indicate that the morphological and structural stability of POVs can be varied through control over the counter-cations. The charging/discharging measurements for the MgV10 indicate a better cyclability and rate performance compared with those of the KNaV10. The structure sustainability and electrochemical properties of POVs can be tuned by the counter-cations. The Mg2+ ions remain stable during repeated Li+ ion insertion/desertion upon discharge/charge cycles, and appeared as a strong supporting-point to keep the structure stable. This is of vital importance to the design of POVs based electrode materials. Furthermore, this work provides useful guidelines for the POVs based electrode optimization in the future, and enriches the in-depth understanding of the correlations among the chemical make-up, the structure sustainability, and the electrochemical properties of the POMs based electrode for rechargeable batteries.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21603162 and 51671145), Tianjin Sci. & Tech. Program (17JCYBJC21500), major projects of new materials of Tianjin city (16ZXCLGX00120), and the Fundamental Research Funds of Tianjin University of Technology.Notes and references
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587–603 CrossRef CAS.
- B. Scrosati, J. Hassoun and Y.-K. Sun, Energy Environ. Sci., 2011, 4, 3287 CAS.
- X. Z. Liu, D. Li, S. Bai and H. S. Zhou, J. Mater. Chem. A, 2015, 3, 15403–15407 CAS.
- X. H. Rui, X. X. Zhao, Z. Y. Lu, H. T. Tan, D. Sim, H. H. Hng, R. Yazami, T. M. Lim and Q. Y. Yan, ACS Nano, 2013, 7, 5637–5646 CrossRef CAS PubMed.
- L. He, S. C. Zhang, X. Wei, Z. J. Du, G. R. Liu and Y. L. Xing, J. Power Sources, 2012, 220, 228–235 CrossRef CAS.
- X. X. L. Li, F. Y. Kang, Y. P. Zheng, X. J. Shi and W. C. Shen, Key Eng. Mater., 2007, 336–338, 463–465 CrossRef CAS.
- L. H. Yu, Y. L. Cao, H. X. Yang, X. P. Ai and Y. Y. Ren, Mater. Chem. Phys., 2004, 88, 353–356 CrossRef CAS.
- F. Jiao, K. M. Shaju and P. G. Bruce, Angew. Chem., Int. Ed., 2005, 117, 6708–6711 CrossRef.
- L. H. Yu, H. X. Yang, X. P. Ai and Y. L. Cao, J. Phys. Chem. B, 2005, 109, 1148–1154 CrossRef CAS PubMed.
- C. X. Wu, L. K. Yan, T. Zhang and Z. M. Su, Inorg. Chem. Front., 2015, 2, 246–253 RSC.
- N. Casan-Pastor and P. Gomez-Romero, Front. Biosci., 2004, 9, 1759–1770 CrossRef CAS.
- M. Genovese and K. Lian, Curr. Opin. Solid State Mater. Sci., 2015, 19, 126–137 CrossRef CAS.
- S. Herrmann, C. Ritchie and C. Streb, Dalton Trans., 2015, 44, 7092–7104 RSC.
- J. Hu, Y. C. Ji, W. Chen, C. Streb and Y.-F. Song, Energy Environ. Sci., 2016, 9, 1095–1101 CAS.
- K. Lu, Z. Y. Hu, Z. H. Xiang, J. Z. Ma, B. Song, J. T. Zhang and H. Y. Ma, Angew. Chem., Int. Ed., 2016, 55, 10448–10452 CrossRef CAS PubMed.
- X. Y. Yang, T. Wei, J.-S. Li, N. Sheng, P.-P. Zhu, J.-Q. Sha, T. Wang and Y. Q. Lan, Inorg. Chem., 2017, 56, 8311–8318 CrossRef CAS PubMed.
- S. Wang, H. L. Li, S. Li, F. Liu, D. Q. Wu, X. L. Feng and L. X. Wu, Chem. – Eur. J., 2013, 19, 10895–10902 CrossRef CAS PubMed.
- Y. C. Ji, L. J. Huang, J. Hu, C. Streb and Y.-F. Song, Energy Environ. Sci., 2015, 8, 776–789 CAS.
- W. Chen, L. J. Huang, J. Hu, T. F. Li, F. F. Jia and Y.-F. Song, Phys. Chem. Chem. Phys., 2014, 16, 19668–19673 RSC.
- D. Ma, L. Y. Liang, W. Chen, H. M. Liu and Y. F. Song, Adv. Funct. Mater., 2013, 23, 6100–6105 CrossRef CAS.
- M. R. Mohammadi and M. Hakimi, Eur. J. Chem., 2012, 9, 43–48 CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2015, 71, 3–8 CrossRef PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- A. Iida and T. Ozeki, Acta Crystallogr., 2004, 60, i43–i46 Search PubMed.
- S. T. Wang, W. Quan, Z. Zhu, Y. Yang, Q. Liu, Y. Ren, X. Y. Zhang, R. Xu, Y. Hong, Z. T. Zhang, K. Amine, Z. L. Tang, J. Lu and J. Li, Nat. Commun., 2017, 8, 627 CrossRef PubMed.
- T. Zhang, H. J. Yue, H. L. Qiu, Y. J. Wei, C. Z. Wang, G. Chen and D. Zhang, Nanotechnology, 2017, 28, 105403 CrossRef PubMed.
- C. H. Jiang, Z. L. Tang, S. T. Wang and Z. T. Zhang, J. Power Sources, 2017, 357, 144–148 CrossRef CAS.
- N. Bucher, S. Hartung, A. Nagasubramanian, Y. L. Cheah, H. E. Hoster and S. Madhavi, ACS Appl. Mater. Interfaces, 2014, 6, 8059–8065 CAS.
- G. Silversmit, D. Depla, H. Poelman, G. B. Marin and R. De Gryse, J. Electron Spectrosc. Relat. Phenom., 2004, 135, 167–175 CrossRef CAS.
- H. Seyama and M. Soma, J. Chem. Soc., Faraday Trans. 1, 1984, 80, 237–248 RSC.
- S. Hartung, N. Bucher, H.-Y. Chen, R. Al-Oweini, S. Sreejith, P. Borah, Y. L. Zhao, U. Kortz, U. Stimming, H. E. Hoster and M. Srinivasan, J. Power Sources, 2015, 288, 270–277 CrossRef CAS.
- H. W. Liu and J. Wang, Ionics, 2010, 16, 379–383 CrossRef CAS.
- J. J. Chen, M. D. Symes, S. C. Fan, M. S. Zheng, H. N. Miras, Q. F. Dong and L. Cronin, Adv. Mater., 2015, 27, 4649–4654 CrossRef CAS PubMed.
- J. L. Liu, Z. Chen, W. M. Xuan, S. Chen, B. W. Zhang, J. Wang, H. H. Wang, B. B. Tian, M. H. Chen, X. F. Fan, Y. Z. Huang, T. C. Sum, J. Y. Lin and Z. X. Shen, ACS Nano, 2017, 11, 6911–6920 CrossRef CAS PubMed.
- W. Kaveevivitchai and A. J. Jacobson, Chem. Mater., 2013, 25, 2708–2715 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7qi00581d |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2017 |