Elastomeric polyethylenes accessible via ethylene homo-polymerization using an unsymmetrical α-diimino-nickel catalyst†‡
Abstract
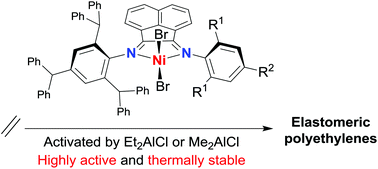
Five types of unsymmetrical bis(arylimino)acenaphthenes, 1-[2,4,6-(CHPh2)3C6H2N]-2-(ArN)C2C10H6 (Ar = 2,6-Me2Ph L1, 2,6-Et2Ph L2, 2,6-i-Pr2Ph L3, 2,4,6-Me3Ph L4 and 2,6-Et2-4-MePh L5), each containing a single N-2,4,6-tribenzhydrylphenyl group, have been prepared and fully characterized. The interaction of L1–L5 with (DME)NiBr2 (DME = 1,2-dimethoxyethane) afforded the corresponding 1 : 1 nickel(II) bromide chelates, LNiBr2 (Ni1–Ni5), in good yield. Distorted tetrahedral geometries are a feature of the X-ray structures of Ni1 and Ni3; broad paramagnetically shifted peaks are seen in the 1H NMR spectra for all the nickel complexes in solution. Upon activation with relatively low amounts of Et2AlCl or Me2AlCl (200–700 equivalents), Ni1–Ni5 exhibited exceptionally high activities for ethylene polymerization (up to 1.07 × 107 g of PE (mol of Ni)−1 h−1), displayed good thermal stability [2.97 × 106 g of PE (mol of Ni)−1 h−1 even at 90 °C] and produced hyperbranched polyethylenes. Dynamic mechanical analysis and stress–strain testing reveal that the polymeric materials possess good elastomeric recovery and high elongation at break, indicating a promising alternative material to thermoplastic elastomers (TPEs).


 Please wait while we load your content...
Please wait while we load your content...