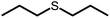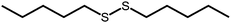Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Robust triplet–triplet annihilation photon upconversion by efficient oxygen scavenging†
Damir
Dzebo
 ,
Kasper
Moth-Poulsen
,
Kasper
Moth-Poulsen
 and
Bo
Albinsson
and
Bo
Albinsson
 *
*
Chalmers University of Technology/Department of Chemistry and Chemical Engineering, 41296 Gothenburg, Sweden. E-mail: balb@chalmers.se; Fax: +46 (0)31 772 38 58; Tel: +46 (0)31 772 30 44
First published on 5th July 2017
Abstract
We hereby present a simple method for reducing the effect of oxygen quenching in Triplet–Triplet Annihilation Upconversion (TTA-UC) systems. A number of commercially available thioethers and one thiol have been tested as singlet oxygen scavengers. Recording of the upconverted emission from a well-studied PdOEP (sensitizer)–DPA (annihilator/emitter) couple has been made over time with steady-state excitation capturing the steady-state kinetics of the TTA-UC process as the solubilized oxygen is depleted by reaction with the scavengers. The efficiency of the TTA-UC process is compared between chemical oxygen scavenging and mechanical removal by inert gas purging or the freeze–pump–thaw method. Selected methods are combined to explore the highest attainable TTA-UC quantum yield. A maximum TTA-UC quantum yield of 21% with the shortest UC onset time was obtained with dimethylthiomethane (DMTM) as the scavenger in an air-saturated solvent and slightly higher quantum yields were obtained in combination with other deoxygenation techniques. Samples containing DMTM displayed little decrease in the quantum yield over four hours of continuous high intensity irradiation, which illustrates the robustness of applying chemical oxygen removal in TTA-UC instead of more time-consuming mechanical processes that usually require specialized equipment.
1. Introduction
Photon upconversion through Triplet–Triplet Annihilation Upconversion (TTA-UC) is a promising technology for the conversion of incoherent low intensity photons to photons with higher frequencies.1 As illustrated in Fig. 1 the process relies on two molecules: a sensitizer (S) and an annihilator (A). The singlet ground state sensitizer (1S) is excited by low energy photons and forms its first excited triplet state (3S*) through rapid inter-system crossing (ISC). The triplet state energy can be transferred to a ground state annihilator (1A) by the process of Triplet Energy Transfer (TET), thus returning the sensitizer to 1S while the annihilator is excited to its triplet excited state (3A*). Two 3A* can subsequently interact and annihilate so that one annihilator accepts the sum of both excited triplet state energies and populates its singlet excited state (1A*) while the other returns to 1A. The 1A* then radiates the upconverted photon at a wavelength shorter than the photon used to excite 1S, as illustrated in Fig. 1.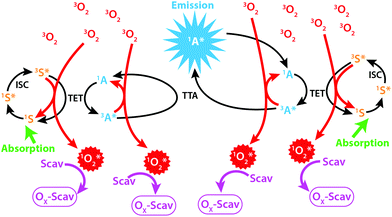 | ||
| Fig. 1 Illustration of the TTA-UC process in the presence of molecular oxygen and a scavenger (Scav). | ||
The TTA-UC process relies on the diffusion of S and A, vital for the intermolecular TET and TTA processes, which is normally achieved in low viscosity solvents such as toluene.2 Since the process relies on diffusion one highly desired property of S and A is long lived excited triplet states so that the probability of intermolecular interactions during the lifetime of the excited triplet states is maximized. This property goes, however, hand in hand with a high sensitivity to species capable of quenching the triplet states. One such species is molecular oxygen (O2)2 which under atmospheric conditions dissolves in toluene to concentrations that almost completely quench the TTA-UC process (Fig. 1).3 The field of TTA-UC has evolved at a fast pace over recent years with one of the major goals being the circumvention of oxygen sensitivity while maintaining a high upconversion quantum yield.1,4 This problem is certainly of practical nature but it is still important to be addressed.
Some of the potential solutions to this problem include high-viscosity matrices with low oxygen permeability.5–8 Similarly, protective coating has been applied to rubbery matrices to isolate the TTA-UC system from oxygen as well as (organic) silica-coating to semi-fluid systems in liposomes.9,10 So far, the highest robustness has been found in a system without solvent where the annihilator has been modified to act as a viscous solvent itself.11 This benefits the contact between the interacting species and allows the TTA-UC process to proceed with rates that exceed the diffusion limit, thus lowering the sensitivity of the diffusion limited O2 quenching. Similar effects have been observed in solid matrices capable of facilitating triplet exciton migration.12,13 Chemical binding of O2 during the TTA-UC process has also been demonstrated by the Baluschev group within the chromophores or also with specially made sacrificial scavenging species.14–16 A similar approach has recently been demonstrated by the Castellano group where polyethylene glycol is used to lower O2 solubility while oleic acid is used as an antioxidant.17
Here we present a convenient way of removing O2 from a sealed TTA-UC system by simply adding commercially available chemical compounds as scavengers while continuously exciting the TTA-UC system. In the beginning of a typical experiment, the TTA-UC process is quenched by O2 while simultaneously the O2 is sensitized to its reactive excited singlet state 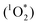 from where an oxidation of the scavenger is possible.14,18,19 This is expected to occur through a nucleophile-like mechanism characteristic of oxidation with singlet excited oxygen, unlike the radical reaction mechanism of triplet ground state oxygen which would require a radical initiator.20–25 The advantage of using commercially available scavengers are several: (i) allowing for usage in larger volumes, (ii) efficient removal of O2 due to the complete TTA-UC process acting as an O2-sensitizer and thus also as a scavenging activator, (iii) permanent depletion of O2 due to an irreversible reaction with the scavenger, (iv) high reproducibility in oxygen removal resulting in higher reproducibility of the TTA-UC efficiency and (v) independence of sample geometry and therefore convenient for applications. The TTA-UC system employed in this study consists of the well known and commercially available palladium(II) octaethylporphyrin (PdOEP) as the sensitizer and 9,10-diphenylanthracene (DPA) as the annihilator.26–28 This study is evaluated and compared against an already published study by the Castellano group who used the same molecules and concentrations (PdOEP/DPA at 5 μM/100 μM) where an UC quantum yield of 16% was reported (defined so that the theoretical maximum ΦUC is 50%).27
from where an oxidation of the scavenger is possible.14,18,19 This is expected to occur through a nucleophile-like mechanism characteristic of oxidation with singlet excited oxygen, unlike the radical reaction mechanism of triplet ground state oxygen which would require a radical initiator.20–25 The advantage of using commercially available scavengers are several: (i) allowing for usage in larger volumes, (ii) efficient removal of O2 due to the complete TTA-UC process acting as an O2-sensitizer and thus also as a scavenging activator, (iii) permanent depletion of O2 due to an irreversible reaction with the scavenger, (iv) high reproducibility in oxygen removal resulting in higher reproducibility of the TTA-UC efficiency and (v) independence of sample geometry and therefore convenient for applications. The TTA-UC system employed in this study consists of the well known and commercially available palladium(II) octaethylporphyrin (PdOEP) as the sensitizer and 9,10-diphenylanthracene (DPA) as the annihilator.26–28 This study is evaluated and compared against an already published study by the Castellano group who used the same molecules and concentrations (PdOEP/DPA at 5 μM/100 μM) where an UC quantum yield of 16% was reported (defined so that the theoretical maximum ΦUC is 50%).27
2. Experimental section
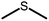
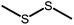
The structures of the sensitizer palladium(II)-octaethylporphyrin (PdOEP) and the annihilator 9,10-diphenylanthracene (DPA) are shown in Fig. 2, while the used scavenger candidates are depicted in Table 1 along with their respective abbreviations. All compounds were used as purchased without any further purification together with PdOEP and DPA in toluene. Two concentrations of the PdOEP/DPA pair are used, 16 μM/500 μM and 5 μM/100 μM, which are referred to as “high” and “low” TTA-UC concentrations, respectively.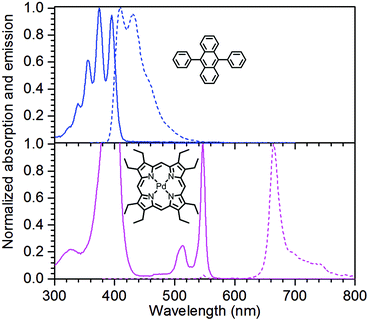 | ||
| Fig. 2 Absorption (solid) and emission (dashed) spectra of the annihilator DPA (top) and the sensitizer PdOEP (bottom) including molecular structures. | ||
Upconversion measurements were performed using a frequency doubled cw Nd:YAG laser (Millenia V, Spectra-Physics) with an output at 532 nm and an average power of 370 mW as the excitation source. The beam diameter was 2.5 mm, determined using burnpaper (ZapIt) and calipers. This gives a photon flux of 2 × 1019 photons per cm2 per s, which is high enough to ensure that TTA-UC emission is linearly dependent on, and the UC quantum yield (ΦUC) independent of, excitation intensity, provided that O2 has been thoroughly depleted through scavenging or otherwise.
Emission was captured using a AvaSpec 2048-2 (Avantes) USB fibre spectrometer with a 532 nm notch filter between the sample and the fibre to protect the spectrometer from the intense scattered excitation light. This, in combination with the presence of the sensitizer in the upconversion sample causes perturbation of the upconverted DPA signal through reabsorption, the magnitude of which can change over time if the sensitizer is degraded. In such a case the depletion of the sensitizer has a negative effect on upconversion as the sensitization step becomes less efficient while simultaneously the reabsorption of the UC signal also decreases. In order to avoid these competing perturbing effects, the spectrum of directly excited DPA was scaled to overlap with the non-perturbed region of the UC signal at 460 nm and was used to calculate the upconversion quantum yields.
The ΦUC was determined relative to Cresyl violet perchlorate (Aldrich) dissolved in methanol with a fluorescence quantum yield (Φf = 0.54)29 using eqn (1)
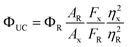 | (1) |
Absorption measurements were performed on a Cary 50 Bio absorption spectrometer and phosphorescence measurements were performed on a Cary Eclipse.
In the initial screening for suitable scavengers, samples were prepared by mixing the prepared TTA-UC solution in toluene with the corresponding scavenger in 2 mL vials with 1 cm internal diameter, a screw cap, a Teflon seal and a parafilm outside, filled so that no air pocket remained. Measurements involving deoxygenation by bubbling of N2 gas (10 min) were conducted in pyrex test tubes with an internal diameter of 1 cm closed with a rubber septum towards the atmosphere and containing a gas pocket volume in the tube corresponding to twice the sample volume. Measurements involving the freeze–pump–thaw degassing procedure were carried out by repeating the procedure 3 times in a medium vacuum of approximately 1 mbar and melt-sealing the samples with a blowtorch in the same kind of sample tube as for N2 purged samples. All samples were stirred during measurements in an attempt to maintain any remaining O2 concentration at equilibrium in the solvent.
For the initial screening of samples that were equilibrated to the atmosphere, the expected initial O2 concentration was 1.74 mM, estimated using Henry's law constant of Htoluene/air = 119 atm M−1. This is under the assumption that the addition of thiols or thioethers does not change the solubility of O2 to any significant degree in the used solvent mixtures compared to pure toluene. The absorption and emission spectra of the TTA-UC system are shown in Fig. 2.
3. Results and discussion
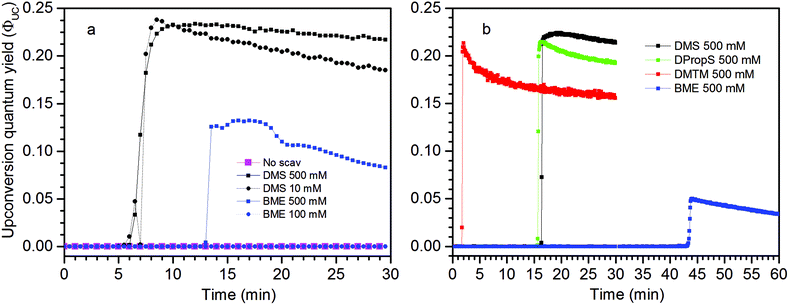
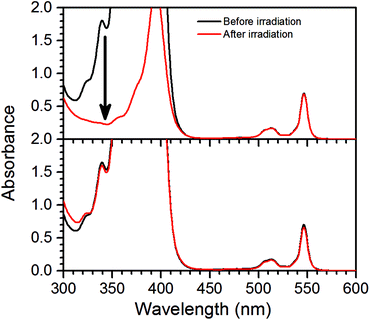
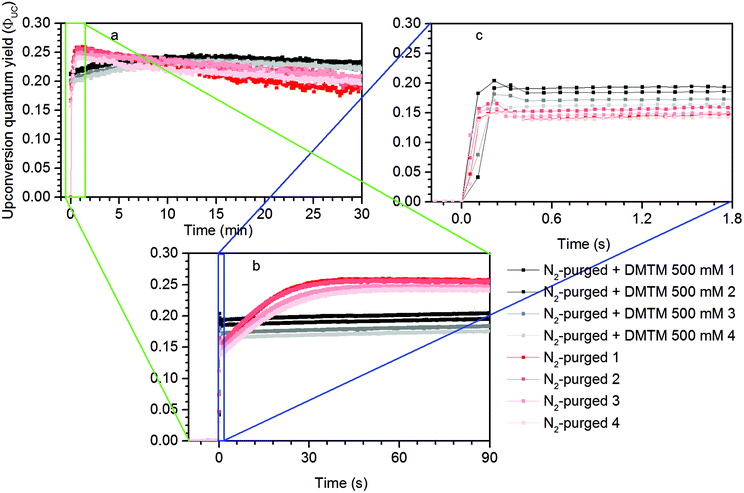
Measurements using a TTA-UC system with high concentration ([PdOEP] = 16 μM and [DPA] = 500 μM) in the presence of 500 mM dimethyl sulfide (DMS) as an oxygen scavenger, showed a substantially increased ΦUC to an impressive value of 24% roughly 7 minutes after the start of irradiation at 532 nm, as seen in Fig. 3a. A sample with the same TTA-UC system without a scavenger produced no UC emission for the duration of the irradiation time (30 min). Fig. 4 illustrates the difference in absorbance of the two samples before and after irradiation. The sample without a scavenger suffered severe photodegradation, primarily visible in the region of the DPA absorption (300–400 nm).A similar value of ΦUC was reached with DMS at a concentration of 10 mM (Fig. 3a). However, the quantum yield dropped more by the end of the measurement which might be due to incomplete oxygen scavenging by the lower concentration of DMS. Degradation was not found to be severe as seen in the absorption spectra before and after irradiation as shown in Fig. S1 in the ESI.†
Using 2-mercaptoethanol (BME), a known oxygen scavenger in the fields of bioscience and microscopy,30 at 500 mM concentration proved not as efficient in removing oxygen. A maximum ΦUC of about 13% was reached after about 13 minutes (Fig. 3a). The late onset of the UC emission can be attributed to a less efficient scavenger and as a consequence the sensitizer and annihilator are being degraded. As the sensitizer and annihilator concentrations decrease, the O2 sensitization decreases and the conditions for the TTA-UC process fundamentally change resulting in a delay of the UC emission onset as well as lowering of ΦUC. Using even lower concentrations of BME (100 mM), no UC emission was detected and absorption measurements reveal severe degradation comparable to the sample without a scavenger (Fig. S3†).
The TTA-UC system with lower concentrations ([PdOEP] = 5 μM and [DPA] = 100 μM; Fig. 3b), corresponding to the figure of merit with reference to that yielded ΦUC = 16%,27 was prepared and the highest ΦUC of 22% was obtained with 500 mM DMS. Indeed the UC emission onset emerges later in time (at around 17 min) which is the result of fewer sensitizers and annihilators serving as sensitizers for O2 and so the scavenging process is slowed down. The maximum ΦUC is still similar to that of the high concentration TTA-UC system which can be attributed to a similar sensitizer/annihilator ratio which is of high importance as it reflects the efficiency of the sensitizer to annihilator Triplet Energy Transfer (TET) process.
Under these conditions dipropyl sulfide (DPropS) was also tested for the O2 scavenging at 500 mM. The UC emission emerges even sooner with DPropS than with DMS at the same concentrations (Fig. 3). Interestingly though, a slightly higher ΦUC and robustness is obtained using DMS with only minor DPA degradation (Fig. S4†) while sooner and not a high ΦUC with lower robustness was obtained with DPropS. In this respect it might seem surprising that there are practically no signs of DPA degradation at all (Fig. S5†) for DPropS.
Dimethylthiomethane (DMTM) produces a much earlier UC emission onset at about 2–3 minutes after the start of irradiation and the TTA-UC system reaches about the same ΦUC as with the other sulfide scavengers with negligible signs of degradation (Fig. S6†), which makes it the best among those scavenger candidates tested here. The DMTM differs from the previously tested scavengers in that it contains two thioether groups. By stoichiometry, two sulfoxide groups may be created in the oxidation reaction with a single O2 which would constitute complete oxidation and scavenging of molecular oxygen.19 Even though the effective concentration of the thioether groups is twice the molecular concentration for DMTM, it is probably not the main reason for the very early UC emission onset seen in Fig. 3b considering that different concentrations of DMS produced a similar onset time as shown in Fig. 3a. However, the structural proximity of the two thioether groups in DMTM (Table 1) may be of importance as the initial intermediate species of the O2 being attached to one of the thioether groups directly has another thioether group nearby to complete the scavenger oxidation. This is not true for DMS and the other similar species containing only one thioether or thiol functional group. In these cases, the initial intermediate persulfoxide species must be long-lived enough to interact with another scavenger in a second diffusion controlled step before complete scavenging of O2 is achieved.31–33 The apparent differences in kinetics during the ΦUC onset shown in Fig. 3 between different scavengers and different concentrations of the same scavenger may be attributed to the dynamic oxidation mechanism of the used scavengers, which is known to depend on the structures, steric effects and concentrations of the sulfides. This scientific topic is well studied but the exact details fall beyond the scope of this investigation.20,24,34–37
The disulfide compounds 1,2-dipentyl disulfide (PDFP), dimethyl disulfide (DMDS) and cyclohexyl disulfide (ChDS) as well as diphenyl sulfide (DPS) proved inefficient as scavengers as no UC emission was observed and instead severe degradation of the TTA-UC system was observed (Fig. S7–S10†) after 30 minutes of irradiation which is not entirely surprising as they are generally known to be less-efficient  scavengers.38 Dibenzyl sulfide (DBS) was also tested and proved inefficient as no UC emission was observed, but without any trace of degradation. Instead, the DPA concentration seemed unchanged and new absorption bands arose in the 300–350 nm region (Fig. S11†), consistent with previous observations.39 A summary of the screening results (Fig. 3b) of all scavenger candidates using the low concentration TTA-UC system is given in Table 1.
scavengers.38 Dibenzyl sulfide (DBS) was also tested and proved inefficient as no UC emission was observed, but without any trace of degradation. Instead, the DPA concentration seemed unchanged and new absorption bands arose in the 300–350 nm region (Fig. S11†), consistent with previous observations.39 A summary of the screening results (Fig. 3b) of all scavenger candidates using the low concentration TTA-UC system is given in Table 1.
An attempt to quantify the O2 concentration in the irradiated samples was made by preparing 16 μM PdOEP solution with 500 mM DMS (no DPA) and irradiating for 30 minutes. The PdOEP phosphorescence lifetime was then measured using low intensity excitation pulses to minimize the effects of triplet–triplet annihilation between the sensitizers.40 The obtained fit (Fig. S15†) revealed a lifetime of 112 μs compared to 770 μs which was obtained using the freeze–pump–thaw method for deoxygenation and where PdOEP is considered unquenched.40 If the bimolecular oxygen quenching rate (kq) is assumed to be diffusion controlled (∼1 × 1010 M−1 s−1 in toluene41), then using Stern–Volmer kinetics in eqn (2)
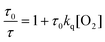 | (2) |
The robustness of the UC signal over 240 minutes with and without the 500 mM DMTM scavenger was investigated in a N2-gas purged sample as this is by far the easiest way of deoxygenating a liquid sample. The data in Fig. 5 clearly demonstrate how sensitive a N2-deoxygenated sample with a septa is for the penetration of O2 and how well the 500 mM DMTM acts as a scavenger under the same conditions. At first, the sample without a scavenger displays a sharp rise in ΦUC probably as a result of some DPA molecules being served as 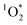 -scavengers. Apart from this initial rise, both samples start at ΦUC of about 21% but the N2-purged sample displays a rapid decrease in ΦUC while the sample that also contains the DMTM scavenger displays a gradual rise in ΦUC to about 23% as the residual and entering O2 is consumed by the scavenger. As the septa does not provide an ideal seal when it comes to O2, the atmospheric O2 may eventually enter the sample and the remaining DMTM cannot compensate completely for the leak. The degree of degradation of the TTA-UC system is displayed in Fig. S12 in the ESI.†
-scavengers. Apart from this initial rise, both samples start at ΦUC of about 21% but the N2-purged sample displays a rapid decrease in ΦUC while the sample that also contains the DMTM scavenger displays a gradual rise in ΦUC to about 23% as the residual and entering O2 is consumed by the scavenger. As the septa does not provide an ideal seal when it comes to O2, the atmospheric O2 may eventually enter the sample and the remaining DMTM cannot compensate completely for the leak. The degree of degradation of the TTA-UC system is displayed in Fig. S12 in the ESI.†
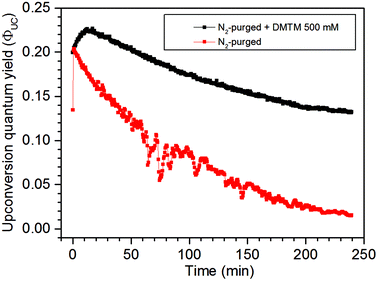 | ||
| Fig. 5 UC emission of the lower concentration TTA-UC system during long irradiation times for N2-deoxygenated samples with and without the 500 mM DMTM scavenger. | ||
The reproducibility of this result was tested at a shorter irradiation time of 30 minutes and repeated four times. A higher time-resolution was also employed to investigate the kinetics features at sub-minute and -second time scales. As seen in Fig. 6a on the minute-scale, in the beginning the N2-purged samples produce the highest ΦUC and drop over time below the ΦUC of the samples containing the DMTM scavenger. However, at higher time resolutions shown in Fig. 6b, it is evident that the DMTM-containing samples produce a more prompt onset with an initial nick in the UC emission trace which is further resolved as shown in Fig. 6c.
In order to isolate the scavenging effect from the possible O2 leaks into the sample, the freeze–pump–thaw (FPT) degassing method was performed with the low concentration TTA-UC system with and without the 500 mM DMTM scavenger. As seen in Fig. 7, again the sample without a scavenger displays a higher ΦUC initially and then drops far below that of the DMTM-containing sample. One possible explanation for this observation might be that there is a slight excess of the DPA annihilator in the sample than optimally required to quench the sensitizer and so a minor amount of DPA may be utilized to scavenge part of the residual O2 in the sealed sample without resulting in a major ΦUC loss. However, the ΦUC of the non-scavenged sample keeps decreasing over time which suggests that there is more O2 in the sample than can be consumed with only excess DPA and so there is a negative effect on the long-time stability of ΦUC. This effect is not nearly as strong with the DMTM-containing sample as the scavenger is being oxidized, and so the scavenged sample attains an almost constant and robust ΦUC for the duration of the high intensity excitation experiment. The degree of component degradation in this experiment can be found in the absorption spectra shown in Fig. S13 and S14 in the ESI.†
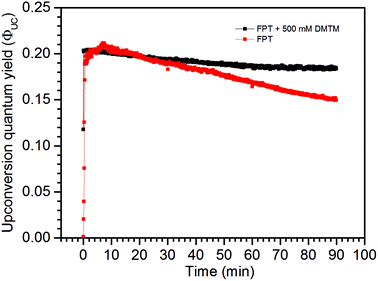 | ||
| Fig. 7 UC emission of two melt-sealed low concentration TTA-UC system samples after 3 FPT cycles to 1 mbar, with and without the 500 mM DMTM scavenger. | ||
4. Conclusion
Introducing oxygen scavengers as a third species in a TTA-UC system may function well in terms of providing robust upconversion as long as the scavenger is more readily oxidized than the TTA-UC components. Of the series of nine tested scavengers, DMTM seems indeed promising as a singlet oxygen scavenger which may not be so surprising as it most likely is oxidized similar to DMS where the major oxidation product is dimethyl sulfoxide (DMSO).33,42 We hypothesize that the DMTM lacks a diffusion controlled step in the full reduction of O2 which is probably the reason for its higher efficiency as a scavenger. Also, the end-product's DMSO-like properties are attractive as DMSO is a well known and often used spectroscopic solvent which fulfills one of the demands for the scavenger oxidation product, i.e. to not interfere with the TTA-UC system.We have shown here that it is possible to use thioether- and thiol-like compounds as a third species in a TTA-UC system in order to chemically remove molecular oxygen. However, it is crucial for long lasting experiments that the samples are well sealed. More specifically, we have demonstrated by using the commercially available DMTM as the O2 scavenger that it is possible to achieve and maintain a high ΦUC by applying a few freeze–pump–thaw cycles with a relatively low vacuum followed by a melt-sealing of the sample. This methodology makes it possible to prepare a high-quality and fully fluid TTA-UC sample in less time and without the need for expensive high-vacuum and specialized equipment.
Acknowledgements
Prof. Jerker Mårtensson is kindly acknowledged for valuable input on the investigated scavengers and scientific discussion. The authors acknowledge financial support from the Swedish Energy Agency (Energimyndigheten), the K. & A. Wallenberg foundation and the Swedish Foundation for Strategic Research.References
- C. E. McCusker and F. N. Castellano, Materials Integrating Photochemical Upconversion, Top. Curr. Chem., 2016, 374, 2–25 CrossRef PubMed.
- T. W. Schmidt and F. N. Castellano, Castellano, Photochemical Upconversion: The Primacy of Kinetics, J. Phys. Chem. Lett., 2014, 5, 4062–4072 CrossRef CAS PubMed.
- W. R. Ware, Oxygen Quenching of Fluorescence in Solution: An Experimental Study of the Diffusion Process, J. Phys. Chem., 1962, 66, 455–458 CrossRef CAS.
- T. F. Schulze and T. W. Schmidt, Photochemical upconversion: Present status and prospects for its application to solar energy conversion, Energy Environ. Sci., 2014, 103–125 Search PubMed.
- C. Li, C. Koenigsmann, F. Deng, A. Hagstrom, C. A. Schmuttenmaer and J.-H. Kim, Photocurrent Enhancement from Solid-State Triplet–Triplet Annihilation Upconversion of Low-Intensity, Low-Energy Photons, ACS Photonics, 2016, 3, 784–790 CrossRef CAS.
- S. H. Lee, M. A. Ayer, R. Vadrucci, C. Weder and Y. C. Simon, Light upconversion by triplet-triplet annihilation in diphenylanthracene-based copolymers, Polym. Chem., 2014, 5, 6898–6904 RSC.
- S. H. Lee, J. R. Lott, Y. C. Simon and C. Weder, Melt-processed polymer glasses for low-power upconversion via sensitized triplet-triplet annihilation, J. Mater. Chem. C, 2013, 1, 5142–5148 RSC.
- S. Baluschev, J. Jacob, Y. S. Avlasevich, P. E. Keivanidis, T. Miteva, A. Yasuda, G. Nelles, A. C. Grimsdale, K. Mullen and G. Wegner, Enhanced operational stability of the up-conversion fluorescence in films of palladium-porphyrin end-capped poly(pentaphenylene), ChemPhysChem, 2005, 6, 1250–1253 CrossRef CAS PubMed.
- A. J. Svagan, D. Busko, Y. Avlasevich, G. Glasser, S. Baluschev and K. Landfester, Photon energy upconverting nanopaper: a bioinspired oxygen protection strategy, ACS Nano, 2014, 8, 8198–8207 CrossRef CAS PubMed.
- S. H. Askes, V. C. Leeuwenburgh, W. Pomp, H. Arjmandi-Tash, S. Tanase, T. Schmidt and S. Bonnet, Water-Dispersible Silica-Coated Upconverting Liposomes: Can a Thin Silica Layer Protect TTA-UC against Oxygen Quenching?, ACS Biomater. Sci. Eng., 2017, 3, 322–334 CrossRef CAS PubMed.
- P. Duan, N. Yanai and N. Kimizuka, Photon upconverting liquids: matrix-free molecular upconversion systems functioning in air, J. Am. Chem. Soc., 2013, 135, 19056–19059 CrossRef CAS PubMed.
- P. Mahato, N. Yanai, M. Sindoro, S. Granick and N. Kimizuka, Preorganized Chromophores Facilitate Triplet Energy Migration, Annihilation and Upconverted Singlet Energy Collection, J. Am. Chem. Soc., 2016, 138, 6541–6549 CrossRef CAS PubMed.
- N. Yanai and N. Kimizuka, Recent emergence of photon upconversion based on triplet energy migration in molecular assemblies, Chem. Commun., 2016, 52, 5354–5370 RSC.
- F. Marsico, A. Turshatov, R. Pekoz, Y. Avlasevich, M. Wagner, K. Weber, D. Donadio, K. Landfester, S. Baluschev and F. R. Wurm, Hyperbranched Unsaturated Polyphosphates as Protective Matrix for Long-Term Photon Upconversion in Air, J. Am. Chem. Soc., 2014, 11057–11064 CrossRef CAS PubMed.
- M. A. Filatov, E. Heinrich, D. Busko, I. Z. Ilieva, K. Landfester and S. Baluschev, Reversible oxygen addition on a triplet sensitizer molecule: protection from excited state depopulation, Phys. Chem. Chem. Phys., 2015, 17, 6501–6510 RSC.
- S. Baluschev, K. Katta, Y. Avlasevich and K. Landfester, Annihilation upconversion in nanoconfinement: solving the oxygen quenching problem, Mater. Horiz., 2016, 3, 478–486 RSC.
- C. Mongin, J. H. Golden and F. N. Castellano, Liquid PEG Polymers Containing Antioxidants: A Versatile Platform for Studying Oxygen-Sensitive Photochemical Processes, ACS Appl. Mater. Interfaces, 2016, 8, 24038–24048 CAS.
- S. M. S. Chauhan, A. Kumar and K. A. Srinivas, Oxidation of thiols with molecular oxygen catalyzed by cobalt(II) phthalocyanines in ionic liquid, Chem. Commun., 2003, 2348–2349 RSC.
- G. A. Bagiyan, I. K. Koroleva, N. V. Soroka and A. V. Ufimtsev, Oxidation of thiol compounds by molecular oxygen in aqueous solutions, Russ. Chem. Bull., 2003, 52, 1135–1141 CrossRef CAS.
- C. Gu, C. S. Foote and M. L. Kacher, Chemistry of singlet oxygen. 35. Nature of intermediates in the photooxygenation of sulfides, J. Am. Chem. Soc., 1981, 103, 5949–5951 CrossRef CAS.
- P. R. Ogilby, Singlet oxygen: there is indeed something new under the sun, Chem. Soc. Rev., 2010, 39, 3181–3209 RSC.
- E. L. Clennan, New Mechanistic and Synthetic Aspects of Singlet Oxygen Chemistry, Tetrahedron, 2000, 56, 9151–9179 CrossRef CAS.
- J. J. Liang, C. L. Gu, M. L. Kacher and C. S. Foote, Chemistry of singlet oxygen. 45. Mechanism of the photooxidation of sulfides, J. Am. Chem. Soc., 1983, 105, 4717–4721 CrossRef CAS.
- B. M. Monroe, Rates of Reaction of Singlet Oxygen with Sulfides, Photochem. Photobiol., 1979, 29, 761–764 CrossRef.
- H. H. Wasserman and J. L. Ives, Singlet Oxygen in Organic-Synthesis, Tetrahedron, 1981, 37, 1825–1852 CrossRef CAS.
- S. Baluschev, T. Miteva, V. Yakutkin, G. Nelles, A. Yasuda and G. Wegner, Up-conversion fluorescence: noncoherent excitation by sunlight, Phys. Rev. Lett., 2006, 97, 143903 CrossRef CAS PubMed.
- R. S. Khnayzer, J. Blumhoff, J. A. Harrington, A. Haefele, F. Deng and F. N. Castellano, Upconversion-powered photoelectrochemistry, Chem. Commun., 2012, 48, 209–211 RSC.
- A. Haefele, J. Blumhoff, R. S. Khnayzer and F. N. Castellano, Getting to the (Square) Root of the Problem: How to Make Noncoherent Pumped Upconversion Linear, J. Phys. Chem. Lett., 2012, 3, 299–303 CrossRef CAS.
- D. Magde, J. H. Brannon, T. L. Cremers and J. Olmsted, Absolute luminescence yield of cresyl violet. A standard for the red, J. Phys. Chem., 1979, 83, 696–699 CrossRef CAS.
- J. Beech, L. Nyberg, J. Fritzsche, F. Westerlund and J. Tegenfeldt, 17th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS, 2013, pp. 5–7.
- E. L. Clennan, Persulfoxide: Key Intermediate in Reactions of Singlet Oxygen with Sulfides, Acc. Chem. Res., 2001, 34, 875–884 CrossRef CAS PubMed.
- F. Jensen, A. Greer and E. L. Clennan, Reaction of Organic Sulfides with Singlet Oxygen. A Revised Mechanism, J. Am. Chem. Soc., 1998, 120, 4439–4449 CrossRef CAS.
- S. M. Bonesi, I. Manet, M. Freccero, M. Fagnoni and A. Albini, Photosensitized oxidation of sulfides: discriminating between the singlet-oxygen mechanism and electron transfer involving superoxide anion or molecular oxygen, Chemistry, 2006, 12, 4844–4857 CrossRef CAS PubMed.
- M. L. Kacher and C. S. Foote, Chemistry of Singlet Oxygen XXVIII. Steric and Electronic Effects on the Reactivity of Sulfides with Singlet Oxygen, Photochem. Photobiol., 1979, 29, 765–769 CrossRef CAS.
- C. S. Foote and J. W. Peters, Chemistry of singlet oxygen. XIV. Reactive intermediate in sulfide photooxidation, J. Am. Chem. Soc., 1971, 93, 3795–3796 CrossRef CAS.
- C. C. Winterbourn and M. B. Hampton, Thiol chemistry and specificity in redox signaling, Free Radicals Biol. Med., 2008, 45, 549–561 CrossRef CAS PubMed.
- M. Kemp, Y. M. Go and D. P. Jones, Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology, Free Radicals Biol. Med., 2008, 44, 921–937 CrossRef CAS PubMed.
- E. L. Clennan, D. Wang, C. Clifton and M. F. Chen, Geometry-Dependent Quenching of Singlet Oxygen by Dialkyl Disulfides, J. Am. Chem. Soc., 1997, 119, 9081–9082 CrossRef CAS.
- F. C. Thyrion, Flash photolysis of aromatic sulfur molecules, J. Phys. Chem., 1973, 77, 1478–1482 CrossRef CAS.
- D. Dzebo, K. Börjesson, V. Gray, K. Moth-Poulsen and B. Albinsson, Intramolecular Triplet–Triplet Annihilation Upconversion in 9,10-Diphenylanthracene Oligomers and Dendrimers, J. Phys. Chem. C, 2016, 120, 23397–23406 CAS.
- N. J. Turro, V. Ramamurthy and J. C. Scaiano, Modern molecular photochemistry of organic molecules, University Science Books, Sausalito, 2010 Search PubMed.
- Y. Sawaki and Y. Ogata, Nucleophilic oxygen atom transfer reactions by persulfoxide and persulfone, J. Am. Chem. Soc., 1981, 103, 5947–5948 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Absorption spectra and kinetics. See DOI: 10.1039/C7PP00201G |
| This journal is © The Royal Society of Chemistry and Owner Societies 2017 |