 Open Access Article
Open Access ArticleEmerging pollutant mixture mineralization by TiO2 photocatalysts. The role of the water medium†
Luca
Rimoldi
*ab,
Daniela
Meroni
*ab,
Ermelinda
Falletta
a,
Valentina
Pifferi
 ab,
Luigi
Falciola
ab,
Giuseppe
Cappelletti
ab and
Silvia
Ardizzone
ab
ab,
Luigi
Falciola
ab,
Giuseppe
Cappelletti
ab and
Silvia
Ardizzone
ab
aDipartimento di Chimica, Università degli Studi di Milano, Via Golgi 19, 20133 Milano, Italy. E-mail: luca.rimoldi@unimi.it; daniela.meroni@unimi.it
bConsorzio Interuniversitario Nazionale per la Scienza e la Tecnologia dei Materiali (INSTM), Via Giusti 9, 50121 Firenze, Italy
First published on 26th October 2016
Abstract
Pharmaceutics and personal care products (PPCPs) are raising growing concern due to their widespread usage and resistance to conventional remediation techniques. Several of them raise significant health and environmental concerns, especially when present in complex mixtures. Due to their chemical resistance, Advanced Oxidation Processes (AOPs) are needed for their complete removal from surface and wastewaters. In the present work, photocatalysis by titanium dioxide (TiO2) under UV and simulated solar irradiation was adopted to degrade tetracycline hydrochloride, paracetamol, caffeine and atenolol, both as single pollutants and in mixtures. All molecules showed high removal and mineralization degrees. Moreover, no interference effects decreased the efficiency of the processes in the case of pollutant mixtures, achieving 60% of mineralization after 6 h. An immobilized TiO2 system was also developed by depositing titania on titanium meshes. A 50% mineralization degree of the pollutant mixture was obtained after 6 h, revealing a suitable efficiency for field applications. Eventually, the impact of the matrix composition on the photocatalytic efficiency was investigated by studying the reaction both in simulated drinking water and in commercial bottled mineral water. The scavenger role played by HCO3− species appears to be dominant in inhibiting the mineralization.
Introduction
In the last few years, pharmaceuticals and personal care products (PPCPs) have attracted much attention as a new class of persistent organic pollutants (POPs).1,2 Among these emerging contaminants, pharmaceuticals, such as antibiotics, beta-blockers, antipyretics and stimulants, represent a notable source of concern due to their biological activity and potential health and environmental effects.3,4 Owing to their wide usage, high excretion rate, persistence and resistance to conventional wastewater treatments, they are nowadays widespread in wastewaters, water effluents, and surface waters, in concentrations ranging from ng L−1 to μg L−1.5,6 Being recalcitrant molecules, they tend to accumulate in the environment7 and conventional treatments are often unable to completely remove them from wastewaters. For example, many pharmaceuticals, such as antibiotics, are not susceptible to degradation by biological treatments.8,9Photocatalysis has been proposed as an effective Advanced Oxidation Process (AOP) for the degradation of this class of compounds in waters.10 This technique may permit not only to remove the contaminant from the polluted system, but also to completely degrade it without giving rise to undesired by-products, which may be even more noxious than the parent compound.11–13 TiO2 is still the most widely used photocatalyst for environmental remediation purposes because of its low cost, abundance, chemical and photochemical stability, and high activity.14–16
Although a great deal of effort has been recently devoted to investigate the efficiency of TiO2-based systems for the photocatalytic degradation of single PPCPs,17–21 many fewer studies deal with the development of effective degradation methods for systems mimicking real effluents.22 Wastewaters usually contain complex pollutant mixtures as well as numerous other organic and inorganic species, which can affect the degradation process through interference and matrix effects. A few studies investigated the photocatalytic degradation of pollutant mixtures in real effluents.23,24 Van Doorslaer et al. compared the photocatalytic efficiency of TiO2 for the degradation of moxifloxacin in hospital effluents, achieving 70% of inhibition with respect to demineralized water, as a result of different combined effects, such as different adsorption, the presence of scavenger species, and formation of complexes.25 Very recently, a detailed scenario on the detrimental effect of several electrolytes on TiO2 photocatalysis was provided by Rioja et al.26 Species such as Cl− and HCO3− have been reported to act as scavengers on TiO2 photocatalysis of dyes and pharmaceuticals.27,28 In order to develop efficient remediation treatments for emerging pollutants, it is thus imperative to get a clearer picture of the complex interactions taking place in pollutant mixtures in real life matrices during the photocatalytic process.
Here, the photodegradation reaction of four PPCPs (Table S1†) by home-made anatase TiO2 is presented. Tests performed by using the commercial Evonik P25 TiO2 were also carried out for the sake of comparison. The target molecules were selected among the emerging pollutants found in the rivers and lakes of Northern Italy,29–31 in order to represent different classes of PPCPs. Tetracycline (TC) is a widely used antibiotic for both animals and humans. Caffeine (CF) is a stimulant in pharmaceutical formulations and is the most widely consumed psychoactive drug. Paracetamol (PC), also known as acetaminophen, is the active ingredient of many analgesic and antipyretic medicaments. Atenolol (AT) is a beta-blocker used in the treatment of cardiovascular diseases and hypertension. Each molecule was studied both independently and in mixtures with other compounds. The adsorption kinetics as well as the photocatalytic removal and mineralization degree were investigated. To the best of our knowledge, the photocatalytic degradation of these four molecules in mixtures has never been reported before. Moreover, the effect of the water medium composition on the photocatalytic efficiency was investigated by tests performed both in simulated drinking water, prepared according to a normed protocol and in commercial bottled mineral water. The feasibility of photocatalytic treatments in real life set-ups was further explored by tests under simulated solar irradiation and with immobilized TiO2 systems.
Experimental section
Photocatalyst preparation
The homemade photocatalyst was prepared according to a procedure reported previously32 aimed at obtaining pure anatase samples (see ESI†). A commercial powder (Evonik P25) was employed for the sake of comparison.An immobilized titania system was prepared by brush casting on both sides of a titanium mesh (15 × 5 cm2) a dispersion of the uncalcined laboratory-made TiO2 particles in a stable sol,33 prepared according to a previously reported procedure.34 The coated mesh was then calcined for 1.5 h at 400 °C under O2 flux (9 NL h−1). The deposition procedure was repeated twice.
Characterization methods
Details about the characterization techniques of the homemade photocatalyst are reported in the ESI.†Photocatalytic activity tests
The photocatalytic degradation of the studied pollutants was carried out under both UV (Jelosil HG500 lamp, effective power density 30 mW cm−2) and simulated solar irradiation (a halogen lamp from Lot Oriel, effective power density 1 mW cm−2 in the range 280–400 nm and 14 mW cm−2 in the range 400–800 nm) in a jacketed batch reactor with a volume of 600 mL.Photocatalytic tests were performed both on single molecules and on pollutant mixtures. Tests in different water media, besides ultrapure water, were performed: simulated drinking water, prepared according to Annex B2 of the Second Protocol of the French Norm NF P41-650 regarding the Specifications for Water Filter Pitchers (Table S2,† column 2), and commercial bottled mineral water selected among the most widespread Italian drinking waters (Table S2,† column 3). Details on the conditions and measurements are reported in the ESI.†
Results and discussion
Sample characterization
The synthesized photocatalyst consisted of pure anatase, as revealed by its XRPD pattern (Fig. S1†). No peaks typical of brookite or rutile were appreciable and an average crystallite size of 7 nm was calculated according to the Scherrer equation.The morphology of the photocatalyst was studied by N2 adsorption–desorption isotherms under subcritical conditions (Fig. S2†), showing a specific surface area of 176 m2 g−1. The oxide appeared to be mesoporous with an H2-type hysteresis loop. The total pore volume was 0.238 mL g−1 with the vast majority of the pores (90%) smaller than 6 nm.
The main parameters characterizing the reference Evonik P25 sample are 50 m2 g−1 as specific surface area and 80% anatase and 20% rutile as phase composition.26
Photocatalytic activity
The samples were tested toward the photocatalytic degradation of four emerging organic pollutants (tetracycline, caffeine, paracetamol and atenolol) found in the water bodies of Northern Italy.29–31 Tetracyclines have been linked to the growth of antibiotic resistant bacterial strains in the Maggiore and Geneva lakes.29,30 Caffeine, a known marker to investigate pollution in surface waters, was detected in many rivers of the Trentino region. Paracetamol is the active ingredient of the bestselling over-the-counter drug in Italy. Atenolol was found in several of the main rivers of Northern Italy.31Dark adsorption curves of the pollutant at the pre-irradiated TiO2 surface (Fig. 1a) showed that adsorption equilibrium was reached in less than 10 min for CF, PC and AT, with limited adsorption (<10%). In contrast, TC strongly adsorbs on TiO2 (ca. 40%), possibly due to its chemical structure (the least soluble among the different tested molecules). The effect of pre-irradiation on the pollutant adsorption was studied by comparing dark adsorption tests on a pre-irradiated sample with adsorption tests on a sample that did not receive irradiation pretreatment. Differences in the adsorption curves were within the experimental error.
 | ||
| Fig. 1 Adsorption (a), disappearance (b) and mineralization (c) as a function of time in single pollutant tests (C0 = 35 mg L−1; 0.5 g L−1 TiO2; ultrapure water matrix). | ||
Photolysis tests of the single molecules under UV light (Fig. S3†) showed molecule disappearance after 6 h and was lower than 20% for CF, PC and AT, while TC reached a slightly higher value (ca. 30%). It is noteworthy that the mineralization degree upon 6 h of UV photolysis was limited to 5–6% for all molecules (Fig. S3,† inset). Incomplete degradation products of PPCPs have been reported to be more noxious than the parent compound.11,13
The photocatalytic degradation curves of the single molecules under UV irradiation are reported in Fig. 1b. TC concentration decreases much faster than the other three molecules, completely disappearing after 120 min (Fig. 1b). It is worth noting that 35 min are sufficient to degrade 90% of this molecule. The other photocatalytic reactions proceed more slowly: CF and PC behaved similarly, being degraded, almost completely, at the end of the tests (>90% for both molecules). AT appeared to be the most recalcitrant molecule, reaching 80% degradation at the end of the reaction time. The reaction rates of pollutant disappearance were evaluated by means of pseudo-first order kinetic constants (Table S3†).
Our results compare well with the literature data, although obtained under different experimental conditions. TC degradation by photocatalysis is reported to proceed successfully also with respect to other emerging pollutants. In the work of Di et al., for example, the photodegradation of TC is much faster than that of another antibiotic (ciprofloxacin) and of an endocrine disrupting compound (BPA).35 The photocatalysis of PC was studied by Rivas et al. in experiments promoted by ozone.36 In this case, the degradation of PC occurs faster than that of caffeine. Similar results were confirmed by Espejo et al.37 AT instead is generally reported to be recalcitrant to photodegradation. Both in mixtures with other beta-blockers or with other emerging pollutants including CF, AT is one of the slowest to be degraded.24
TOC measurements showed that all pollutants gave rise to mineralization under the selected conditions (Fig. 1c). TC almost completed photocatalytic oxidation to CO2, water and ammonium salts within the irradiation time (93%). PC showed a good capability to reach complete oxidation (77%), notwithstanding the much slower degradation kinetics with respect to TC (Fig. 1b and Table S3†). AT showed a final mineralization degree higher than 50%. Surprisingly, CF showed the slowest mineralization trend, although the molecule disappears faster than AT. The purinic moiety in the CF structure probably disfavoured a process of complete oxidation. Despite the slowness of the process, the mineralization was not completely inhibited, as shown by the positive trend with respect to time. These latter results are in agreement with the work of Dalmázio et al.,38 who suggested the generation of persistent intermediates of CF degradation after a fast initial pollutant oxidation.
As a further step, the photocatalytic degradation of mixtures of the selected pollutants was studied under different conditions, i.e. with and without the addition of electrolytes, under UV and simulated solar irradiation, with a slurry or an immobilized photocatalyst.
In tests with ultrapure water and TiO2 slurry under UV irradiation, the molecules in the mixture showed a disappearance trend fully comparable with that shown by single molecule tests. As a representative example, Fig. S4† shows a comparison of the disappearance of CF as a single molecule and in the mixture. ESI-MS spectra of the mixture as a function of irradiation time (Fig. 2), mirror the degradation sequence shown by the single molecules: TC disappears in a short-time scale, followed by PC, while even in the mixture CF disappears faster than AT. The mixture showed, also, a good final mineralization degree (Fig. 3). After a steady decreasing trend, at the end of the photocatalytic test, a mineralization degree of 60% was achieved (Fig. S5†). For the sake of comparison, Fig. S5† reports tests with a well-known commercial oxide (Evonik P25) also. The commercial powder shows a better mineralization of the pollutant mixture, possibly also due to its better dispersibility and stability in water. However, these characteristics make its removal from the suspension lengthy and complex (see the Experimental section), decreasing the global process efficiency22 and introducing a possible source of error in the analytical detection.
 | ||
| Fig. 2 ESI-MS spectra of the photocatalytic tests with pollutant mixtures in ultrapure water at t = 0 (a), t = 2 h (b) and t = 5 h (c). | ||
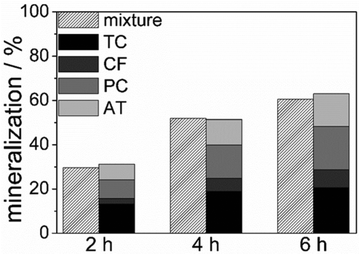 | ||
| Fig. 3 Comparison of the mineralization degree of the tests in a mixture (left) and that of single pollutant tests (right), as a function of irradiation time (ultrapure water, home-made TiO2). | ||
Fig. 3 shows a comparison of the mineralization degree obtained by homemade TiO2 in the test in mixtures with that of tests with single pollutants (using the same initial pollutant concentration) as a function of irradiation time. At each reaction time, the left histogram represents the mineralization of the pollutant mixture measured by TOC determinations, while the right bars report the mineralization degree of each pollutant measured during single pollutant photocatalytic tests. The comparison between the two sets of data suggests that the mineralization rate of the mixture is fully comparable to the sum of the single pollutant ones. Fig. 3, together with results in Fig. 2 and S4,† suggest that there are neither synergistic effects among the different molecules nor detrimental competition for the oxide surface sites or reactive radicals.
The tests using ultrapure water as a solvent were compared with tests using both simulated drinking water, prepared according to a standard protocol, and commercial bottled mineral water (Table S2†). The study of the effect of electrolytes on the photocatalytic activity has recently emerged as a crucial aspect in developing remediation treatments applicable to real effluents.22 Electrolytes can adsorb onto the oxide surface, leading to a decrease of TiO2 efficiency due to a competition for its active sites with the pollutant molecules.27,28 Furthermore, some electrolytes have been reported to act as radical scavengers.26Fig. 4 shows the effect of the presence of inorganic salts on the mineralization curve of photocatalytic tests of the pollutant mixture. In the case of the simulated drinking water, mineralization decreases by about one third with respect to ultrapure water, although a 40% mineralization is achieved at the end of the test. An adsorption competition with inorganic compounds seems limited (Table S4,† column 3), even in the case of TC where the adsorption of the molecule is significant. Interestingly, tests with single pollutants in this simulated water matrix (Table S4,† column 4) show different behaviours: both PC and AT show a marked decrease in the molecule disappearance, whereas the disappearance of both TC and CF is not appreciably inhibited. Again, as in the case of distilled water, neither synergistic nor detrimental competition effects are appreciable among the different molecules in the presence of relatively high concentrations of different electrolytes (Fig. S6†). All molecules appear to be mineralized to a lower extent in the presence of electrolytes, except for CF. This molecule, which is the most recalcitrant to mineralization among the here studied pollutants, is mineralized to a slightly larger extent in the presence of the electrolytes, possibly reflecting the formation of different intermediate species. The general marked decrease of the mineralization might be due to a scavenging effect of the electrolytes toward OH˙ radicals, which are involved in the photocatalytic degradation of all the studied molecules.32,40–42
In the case of the commercial bottled water, the mineralization decrease is much larger (about two thirds) with respect to ultrapure water. The two water samples (Table S2†) show comparable conductivity and pH, while they differ in saline components: the bottled water shows a bicarbonate content three-times larger than the simulated one, together with a lower content in sulphates and chlorides. The comparison between these two sets of data is evidence of a relevant role played by the content of carbonates/bicarbonates in the mixture. Their role as scavengers of ˙OH radicals can be suggested also on the grounds of literature results.39 Indeed, Rioja et al.26 attributed the drastic decrease in the disappearance of clofibric acid, in the case of bottled mineral water, to the competition played by inorganic ions for free radicals.
The mineralization reaction was also studied under simulated solar irradiation thanks to a lamp whose emission extends for ca. 5% in the UV region.16 Although the photocatalytic reaction appeared much slower than under UV irradiation, a limited degree of mineralization was induced, achieving 8.5% after 7 h of irradiation. It should be underlined that the photocatalyst was not promoted in any way to favour light absorption in the visible region and that the effective power density of the lamp was much lower than in UV tests (see the Experimental section). Hence, only the UV tail of the lamp could activate the photocatalytic properties, since anatase TiO2, having a band gap of 3.2 eV, has an absorption edge of 386 nm.
Finally, the photocatalytic degradation of the pollutant mixture was studied using a TiO2-immobilized system. Immobilized systems are often studied for photocatalytic purposes as devices able to be up-scaled for field applications. The removal of the photocatalyst from the treated effluent stands out as one of the most crucial, difficult and expensive operations following water treatment, above all in the case of nanomaterials. In contrast, immobilizing the photocatalyst avoids removing the powder from the water basin, provided that a robust, stable and efficient device is developed. In our case, a homogeneous and stable TiO2 layer, prepared from the laboratory-made TiO2 powder and a stable TiO2 sol, was deposited on a titanium mesh (Fig. S7†). The mineralization curve as a function of the irradiation time during tests with the immobilized system is reported in Fig. 4. Despite the decrease of the surface area due to the process of immobilization, the mineralization curve shows a good photocatalytic performance (50% mineralization after 6 h), without any loss of efficiency throughout the duration of the test. The ease of preparation, applicability to a broad range of substrate types and geometries, and high activity make this immobilization method a valid alternative for field applications.
Conclusions
Four emerging pollutants selected among the main contaminants found in Northern Italy surface waters, were subjected to photocatalytic treatments, both singularly and in mixtures, under several different conditions. Both UV and simulated solar irradiation were employed using either nanosized TiO2 suspensions or homemade immobilized systems. The role played by different water media was also investigated in detail.For all compounds high degradation degrees were achieved in the case of TiO2 slurries in ultrapure water: tetracycline removal occurred on a shorter-time scale (90% after 35 min), while paracetamol, caffeine and atenolol reached 80–90% disappearance after 6 h with slower kinetics. Mineralization was obtained for all molecules as evidence of complete transformation to harmless compounds. Caffeine was found to be the slowest to be mineralized, thus suggesting the production of highly recalcitrant reaction intermediates responsible for low mineralization degrees.
The degradation of the pollutants proceeded in the mixture with the same sequence observed in single molecule tests. Moreover, no lowering of the mineralization degree was observed, thus excluding the occurrence of interference or competition effects, typical of pollutant mixtures. However, it cannot be excluded that at trace or ultra-trace levels differences in the relative mineralization degrees might arise.
The degradation of a mixture of the four contaminants was also studied in a simulated drinking water matrix, by addition of selected electrolytes to ultrapure water, and in a fortified real water matrix (bottled mineral water). An inhibitory effect related to the presence of electrolytes was observed in both cases. However, the studied pollutants exhibited a different behaviour when tested singularly in the presence of electrolytes. Despite the presence of notable amounts of electrolytes with high affinity for oxide surfaces (e.g., sulphates), all of the molecules showed a limited decrease in dark adsorption at the TiO2 surface in simulated drinking water. Tests with mineral water with comparable pH and conductivity but different electrolyte composition, seems to rule out the effect of pH and ionic strength as possible causes of the overall inhibitory effect. The observed changes in the reaction kinetics seem mainly related to the presence of bicarbonates/carbonates, which might act as OH˙ scavengers according to the mechanism:
| HCO3− + ˙OH → CO3˙− + H2O | (1) |
The generated carbonate radical anions have an oxidation potential less positive than that of ˙OH radicals. A modification of the reaction pathway of the different molecules can thus be expected in the presence of electrolytes.
Although our photocatalyst was not promoted to absorb in the visible region, tests under solar irradiation showed a slow but steady increase in time of the pollutant mineralization. This observation supports the absence of strongly adsorbing recalcitrant intermediates, which could have deactivated the surface of the photocatalyst under low irradiation conditions.
Eventually, we tested an immobilized-TiO2 system prepared by depositing TiO2 nanostructured particles and a TiO2 sol on titanium meshes. This system gave rise to 50% mineralization of the pollutant mixture. Considering the relevant loss of surface area following immobilization, the device photocatalytic efficiency was high, revealing a suitable efficiency for field applications.
Acknowledgements
This work has been supported by Fondazione Cariplo (Italy), grant no. 2014-1285.References
- T. Heberer, Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data, Toxicol. Lett., 2002, 131, 5 CrossRef CAS PubMed.
- R. P. Schwarzenbach, The Challenge of Micropollutants in Aquatic Systems, Science, 2006, 313, 1072 CrossRef CAS PubMed.
- A. B. A. Boxall, M. A. Rudd, B. W. Brooks, D. J. Caldwell, K. Choi, S. Hickmann, E. Innes, K. Ostapyk, J. P. Staveley and T. Verslycke, et al., Pharmaceuticals and Personal Care Products in the Environment: What Are the Big Questions?, Environ. Health Perspect., 2012, 120, 1221 CrossRef PubMed.
- B. D. Blair, J. P. Crago, C. J. Hedman and R. D. Klaper, Pharmaceuticals and personal care products found in the Great Lakes above concentrations of environmental concern, Chemosphere, 2013, 93, 2116 CrossRef CAS PubMed.
- E. N. Evgenidou, I. K. Konstantinou and D. A. Lambropoulou, Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: A review, Sci. Total Environ., 2015, 505, 905 CrossRef CAS PubMed.
- D. J. Lapworth, N. Baran, M. E. Stuart and R. S. Ward, Emerging organic contaminants in groundwater: A review of sources, fate and occurrence, Environ. Pollut., 2012, 163, 287 CrossRef CAS PubMed.
- T. Deblonde, C. Cossu-Leguille and P. Hartemann, Emerging pollutants in wastewater: A review of the literature, Int. J. Hyg. Environ. Health, 2011, 214, 442 CrossRef CAS PubMed.
- R. A. Palominos, M. A. Mondaca, A. Giraldo, G. Peñuela, M. Pérez-Moya and H. D. Mansilla, Photocatalytic oxidation of the antibiotic tetracycline on TiO2 and ZnO suspensions, Catal. Today, 2009, 144, 100 CrossRef CAS.
- X. D. Zhu, Y. J. Wang, R. J. Sun and D. M. Zhou, Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2, Chemosphere, 2013, 92, 925 CrossRef CAS PubMed.
- F. Mazille, T. Schoettl, N. Klamerth, S. Malato and C. Pulgarin, Field solar degradation of pesticides and emerging water contaminants mediated by polymer films containing titanium and iron oxide with synergistic heterogeneous photocatalytic activity at neutral pH, Water Res., 2010, 44, 3029 CrossRef CAS PubMed.
- R. Shen and S. A. Andrews, Demonstration of 20 pharmaceuticals and personal care products (PPCPs) as nitrosamine precursors during chloramine disinfection, Water Res., 2011, 45, 944 CrossRef CAS PubMed.
- A. O. Kondrakov, A. N. Ignatev, F. H. Frimmel, S. Bräse, H. Horn and A. I. Revelsky, Formation of genotoxic quinones during bisphenol A degradation by TiO2 photocatalysis and UV photolysis: A comparative study, Appl. Catal., B, 2014, 160–161, 106 CrossRef CAS.
- W.-K. Wang, J.-J. Chen, M. Gao, Y.-X. Huang, X. Zhang and H.-Q. Yu, Photocatalytic degradation of atrazine by boron-doped TiO2 with atunable rutile/anatase ratio, Appl. Catal., B, 2016, 195, 69 CrossRef CAS.
- S. Malato, Removal of Emerging Contaminants in Waste-water Treatment: Removal by Photo-catalytic Processes, in Handbook of Environmental Chemistry, Springer, Berlin, 2008, p. 177 Search PubMed.
- L. Rimoldi, C. Ambrosi, G. Di Liberto, L. Lo Presti, M. Ceotto, C. Oliva, D. Meroni, S. Cappelli, G. Cappelletti, G. Soliveri and S. Ardizzone, Impregnation versus Bulk Synthesis: How the Synthetic Route Affects the Photocatalytic Efficiency of Nb/Ta:N Codoped TiO2 Nanomaterials, J. Phys. Chem. C, 2015, 119, 24104 CAS.
- A. Antonello, G. Soliveri, D. Meroni, G. Cappelletti and S. Ardizzone, Photocatalytic remediation of indoor pollution by transparent TiO2 film, Catal. Today, 2014, 230, 35 CrossRef CAS.
- Z. Pan, E. A. Stemmler, H. J. Cho, W. Fan, L. A. LeBlanc, H. H. Patterson and A. Amirbahman, Photocatalytic degradation of 17α-ethinylestradiol (EE2) in thepresence of TiO2-doped zeolite, J. Hazard. Mater., 2014, 279, 17 CrossRef CAS PubMed.
- W. Lin, H. Zheng, P. Zhang and T. Xu, Pt deposited TiO2 films with exposed {001} facets for photocatalytic degradation of a pharmaceutical pollutant, Appl. Catal., A, 2016, 521, 75 CrossRef CAS.
- M. R. Eskandarian, H. Choi, M. Fazli and M. H. Rasoulifard, Effect of UV-LED wavelengths on direct photolytic and TiO2 photocatalytic degradation of emerging contaminants in water, Chem. Eng. J., 2016, 300, 414 CrossRef CAS.
- C. Martínez, M. Canle L., M. I. Fernández, J. A. Santaballa and J. Faria, Aqueous degradation of diclofenac by heterogeneous photocatalysis using nanostructured materials, Appl. Catal., B, 2011, 107, 110 CrossRef.
- M. J. Arlos, M. M. Hatat-Fraile, R. Liang, L. M. Bragg, N. Y. Zhou, S. A. Andrews and M. R. Servos, Photocatalytic decomposition of organic micropollutants using immobilized TiO2 having different isoelectric points, Water Res., 2016, 101, 351 CrossRef CAS PubMed.
- J. Carbajo, M. Jiménez, S. Miralles, S. Malato, M. Faraldos and A. Bahamonde, Study of application of titania catalysts on solar photocatalysis: Influence of type of pollutants and water matrices, Chem. Eng. J., 2016, 291, 64 CrossRef CAS.
- F. F. Sodré, M. A. F. Locatelli and W. F. Jardim, Occurrence of Emerging Contaminants in Brazilian Drinking Waters: A Sewage-To-Tap Issue, Water, Air, Soil Pollut., 2010, 206, 57 CrossRef.
- L. Prieto-Rodriguez, S. Miralles-Cuevas, I. Oller, A. Agüera, G. L. Puma and S. Malato, Treatment of emerging contaminants in wastewater treatment plants (WWTP) effluents by solar photocatalysis using low TiO2 concentrations, J. Hazard. Mater., 2012, 211–212, 131 CrossRef CAS PubMed.
- X. Van Doorslaer, J. Dewulf, J. De Maerschalk, H. Van Langenhove and K. Demeestere, Heterogeneous photocatalysis of moxifloxacin in hospital effluent: Effect of selected matrix constituents, Chem. Eng. J., 2015, 261, 9 CrossRef CAS.
- N. Rioja, S. Zorita and F. J. Peñas, Effect of water matrix on photocatalytic degradation and general kinetic modeling, Appl. Catal., B, 2016, 180, 330 CrossRef CAS.
- N. Klamerth, N. Miranda, S. Malato, A. Agüera, A. R. Fernández-Alba, M. I. Maldonado and J. M. Coronado, Degradation of emerging contaminants at low concentrations in MWTPs effluents with mild solar photo-Fenton and TiO2, Catal. Today, 2009, 144, 124 CrossRef CAS.
- T. Aarthi, P. Narahari and G. Madras, Photocatalytic degradation of Azure and Sudan dyes using nano TiO2, J. Hazard. Mater., 2007, 149, 725 CrossRef CAS PubMed.
- Agenzia Italiana del Farmaco AIFA, Rapporto sull'uso dei farmaci antibiotici Analisi del consumo territoriale nelle regioni italiane, 2009 Search PubMed.
- N. Czekalski, T. Berthold, S. Caucci, A. Egli and H. Bürgmann, Increased levels of multiresistant bacteria and resistance genes after wastewater treatment and their dissemination into Lake Geneva, Switzerland, Front. Microbiol., 2012, 3, 1 Search PubMed.
- E. Zuccato, D. Calamari, M. Natangelo and R. Fanelli, Presence of therapeutic drugs in the environment, Lancet, 2000, 355, 1789 CrossRef CAS.
- L. Rimoldi, D. Meroni, G. Cappelletti and S. Ardizzone, Green and low cost tetracycline degradation processes by nanometric and immobilized TiO2 systems, Catal. Today, 2016 DOI:10.1016/j.cattod.2016.08.015.
- G. Maino, D. Meroni, V. Pifferi, L. Falciola, G. Soliveri, G. Cappelletti and S. Ardizzone, Electrochemically assisted deposition of transparent, mechanically robust TiO2 films for advanced applications, J. Nanopart. Res., 2013, 15, 2087 CrossRef.
- G. Soliveri, V. Sabatini, H. Farina, M. A. Ortenzi, D. Meroni and A. Colombo, Double side self-cleaning polymeric materials: The hydrophobic and photoactive approach, Colloids Surf., A, 2015, 483, 285 CrossRef CAS.
- J. Di, J. Xia, Y. Ge, H. Li, H. Ji, H. Xu, Q. Zhang, H. Li and M. Li, Novel visible-light-driven CQDs/Bi2WO6 hybrid materials with enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight, Appl. Catal., B, 2015, 168–169, 51 CrossRef CAS.
- F. J. Rivas, F. J. Beltrán and A. Encinas, Removal of emergent contaminants: Integration of ozone and photocatalysis, J. Environ. Manage., 2012, 100, 10 CrossRef CAS PubMed.
- A. Espejo, F. J. Beltrán, F. J. Rivas, J. F. García-Araya and O. Gimeno, Iron-based catalysts for photocatalytic ozonation of some emerging pollutants of wastewater, J. Environ. Sci. Health, Part A, 2015, 50, 553 CAS.
- I. Dalmázio, L. S. Santos, R. P. Lopes, M. N. Eberlin and R. Augusti, Advanced Oxidation of Caffeine in Water: On-Line and Real-Time Monitoring by Electrospray Ionization Mass Spectrometry, Environ. Sci. Technol., 2005, 39, 5982 CrossRef.
- M. Krivec, R. Dillert, D. W. Bahnemann, A. Mehle, J. Štrancar and G. Dražić, The nature of chlorine-inhibition of photocatalytic degradation of dichloroacetic acid in a TiO2-based microreactor, Phys. Chem. Chem. Phys., 2014, 16, 14867 RSC.
- R. R. N. Marques, M. J. Sampaio, P. M. Carrapiço, C. G. Silva, S. Morales-Torres, G. Dražić, J. L. Faria and A. M. T. Silva, Photocatalytic degradation of caffeine: Developing solutions for emerging pollutants, Catal. Today, 2013, 209, 108 CrossRef CAS.
- E. Moctezuma, E. Leyva, C. A. Aguilar, R. A. Luna and C. Montalvo, Photocatalytic degradation of paracetamol: Intermediates and total reaction mechanism, J. Hazard. Mater., 2012, 243, 130 CrossRef CAS PubMed.
- H. Yang, T. An, G. Li, W. Song, W. J. Cooper, H. Luo and X. Guo, Photocatalytic degradation kinetics and mechanism of environmental pharmaceuticals in aqueous suspension of TiO2: A case of β-blockers, J. Hazard. Mater., 2010, 179, 834 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Chemical structures of the molecules, experimental procedures and characterization methods, simulated drinking and bottled mineral water characteristics, XRPD pattern, adsorption–desorption isotherm and pore size distribution of the photocatalyst, data from photolysis tests, pseudo-first-order kinetic constants and final mineralization degrees for single pollutant degradation tests, comparison between caffeine degradation alone and in mixture, mineralization curve of the pollutant mixture obtained adopting Evonik P25 catalyst, adsorption degrees and pseudo-first-order kinetic constants for the single pollutant tests in ultrapure and simulated waters, mineralization degrees for pollutant mixture tests in ultrapure and simulated drinking waters in comparison with single pollutant tests and SEM images of the deposited mesh. See DOI: 10.1039/c6pp00214e |
| This journal is © The Royal Society of Chemistry and Owner Societies 2017 |

