Functional group manoeuvring for tuning stability and reactivity: synthesis of cicerfuran, moracins (D, E, M) and chromene-fused benzofuran-based natural products†
Abstract
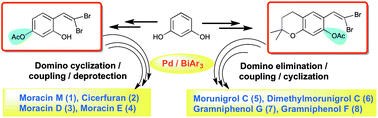
The protecting group manoeuvring as a strategy was applied for tuning the stability and reactivity of 4-(2,2-dibromovinyl)benzene-1,3-diol (12a) and 6-(2,2-dibromovinyl)-2,2-dimethylchroman-7-ol (22) in the domino synthesis of benzofuran-based natural products (1–8). The functional group demands and their impact on the reactivity driven by electronic effects were successfully managed by varying the protecting groups with substituted gem-dibromovinylphenols in domino couplings and triarylbismuth reagents under palladium-catalyzed conditions. This approach paved the way for the synthesis of moracin M (1) and cicerfuran (2), and the first time synthesis of moracin D (3) and moracin E (4) along with chromene-fused benzofuran-based natural products (5–8) in overall good yields.


 Please wait while we load your content...
Please wait while we load your content...