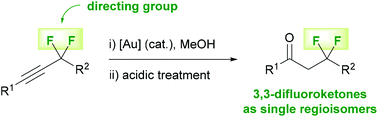Jean-Denys Hamel, Tatsuru Hayashi, Mélissa Cloutier, Paul R. Savoie, Olivier Thibeault, Meggan Beaudoin and Jean-François Paquin
Org. Biomol. Chem., 2017,15, 9830-9836
DOI:
10.1039/C7OB02406A,
Paper