Open Access Article
Open Access ArticleA colorimetric competitive displacement assay for the evaluation of catalytic peptides†
Anneliese M. M.
Gest
 ,
Erika M.
Aguiluz
,
Malik T.
Mays
,
Xinyu
Liu
,
Eliza K.
Neidhart
and
Leah S.
Witus
,
Erika M.
Aguiluz
,
Malik T.
Mays
,
Xinyu
Liu
,
Eliza K.
Neidhart
and
Leah S.
Witus
 *
*
Department of Chemistry, Macalester College, 1600 Grand Ave., Saint Paul, MN 55105, USA. E-mail: lwitus@macalester.edu
First published on 28th November 2017
Abstract
An indicator displacement assay has been adapted to detect the diol products of the aldol reaction between 4-nitrobenzaldehyde and hydroxyacetone in crude reaction mixtures. This provides a rapid colorimetric method of detecting product formation and thus evaluating potential catalysts, which is demonstrated using multiple catalytic peptides.
Peptides are an appealing class of catalyst as they bridge the gap between enzymes and small molecule organocatalysts, sharing some of the complexity of the former and the synthetic accessibility and robustness of the latter. Peptide catalysts have been investigated for a variety of synthetic transformations, including Michael additions, epoxidations, acylations, and direct aldol additions.1–4 Aldol reactions are an important synthetic method of carbon–carbon bond formation, and the aldol product is a structural motif found in a variety of natural products.5 While individual amino acids such as proline are known to catalyze direct aldol reactions,6,7 short peptides have been developed as catalysts as a way to access modular, tuneable catalysts with increased activity and stereoselectivity under mild reaction conditions.8–12
Despite inspiration from aldolase enzymes,11,13 the rational design of catalytic peptide sequences remains difficult for aldol reactions, as well as for other reactions. Multiple groups have found that β-turn-nucleating residues help create secondary structure to serve as a pocket for catalysis in minimal peptides.9,10,14–16 However, the effect of residue on secondary structure and thus catalysis is still difficult to predict, and it has been found that small structural modifications of a lead sequence can result in significant modulations of catalytic activity.15,17 Researchers have often turned to combinatorial methods for the identification of catalytic sequences,18–22 and once leads are identified, derivatives are synthesized to optimize catalysis.14,17 Given the modular nature of peptide synthesis, it is straight-forward to synthesize a large number of analogues to study the effects of sequence on catalysis; however, catalyst evaluation via traditional techniques such as chromatography can become a limiting step in these and in combinatorial screening studies. Therefore, there is interest in rapid methods of catalyst assessment, which have included the use of fluorescent pH sensors23 and co-immobilization of substrates and potential catalysts.24,25 There is utility in developing additional, more generalizable, colorimetric methods. Presented herein is an assay to selectively detect the product of the aldol reaction between 4-nitrobenzaldehyde and hydroxyacetone in crude reaction mixtures based on colorimetric competitive displacement. We demonstrate that this method can rapidly and efficiently differentiate between excellent, good, and noncatalytic peptide sequences using visible light absorption.
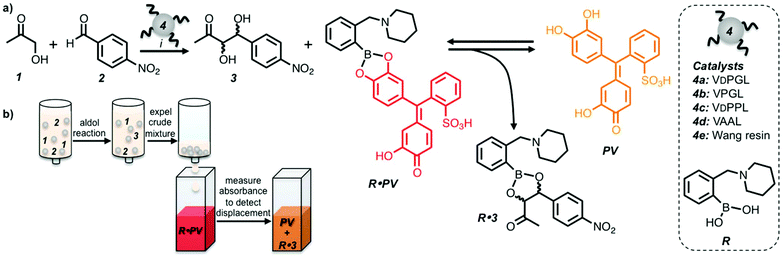
In order to monitor aldol reactions using visible light spectroscopy, we modified an indicator displacement assay (IDA).26–28 IDAs have been used to detect small molecule analytes in solution, based on the competitive displacement of an indicator dye from a receptor compound by the analyte of interest, resulting in a spectroscopic change in the indicator from the receptor-bound to unbound state.27 The Anslyn group has made prodigious use of aryl boronic acid receptors that associate with catechol dyes via reversible boronic ester bonds.29–31 IDAs that sense diols using these components operate based on the detectable blueshift in the catechol dye's absorbance when the diol analyte displaces the catechol dye from the boronic acid receptor. The Anslyn group demonstrated that IDA-based detection of a reaction product can be used to study catalytic ligands for the Sharpless asymmetric dihydroxylation reaction,29 and there is the potential to expand this concept to the study of other reactions and catalysts, which would be particularly powerful if used to evaluate crude reaction mixtures. Herein, we sought to apply a boronic acid host/catechol dye sensing system towards detection of the α,β-dihydroxy ketone product (3) of the aldol reaction between 4-nitrobenzaldehyde (1) and hydroxyacetone (2) in crude reaction mixtures in order to use the dye displacement as a facile, rapid, and inexpensive way to qualitatively evaluate potential peptide catalysts.
The method by which competitive colorimetric displacement can be used for the detection of aldol reaction conversion and thus catalyst evaluation is shown in Scheme 1. To use this assay to evaluate a potentially catalytic peptide sequence (4a–e) the peptide, attached to the solid phase resin, is combined with aldol donor hydroxyacetone and aldol acceptor 4-nitrobenzaldehyde. After incubation, the formation of diol product 3 can be detected using visible light absorption spectroscopy based on the displacement of pyrocatechol violet, PV. This is achieved by expelling the crude reaction mixture from the solid-support bound catalytic species and adding it to a solution of the boronic acid receptor-pyrocatechol violet dye complex, R·PV. The presence of diol product 3 in the crude reaction mixture forms an R·3 complex, displacing the PV dye from the receptor and resulting in a decrease of absorbance at 520 nm. This spectroscopic change can be used to calculate the concentration of product in the crude reaction mixture using a standard curve. In this work, the reaction conversion calculated by this method was compared to the conversion as calculated by crude 1H NMR for five species – four potential catalytic peptides (VDPGL, VPGL, VDPPL, and VAAL) and unfunctionalized Wang resin – serving to validate this competitive displacement assay as a rapid method of evaluating catalytic species for aldol reactions.
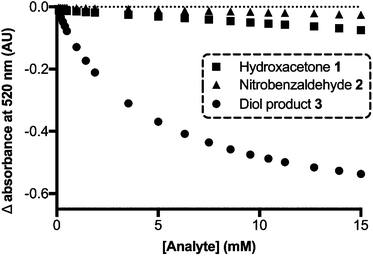
The receptor molecule, R, was synthesized through a reductive amination of 2-formylphenylboronic acid with piperidine following literature protocols.30 To confirm the formation of a complex between PV and R, a titration was performed in which R was added to the dye and the absorbance was monitored. Using the change in absorbance at 520 nm, the dissociation constant was calculated to be 112 μM (Fig. S1 and S2 in the ESI†).‡ This confirmed that PV has distinct absorption maxima when free in solution (λmax = 443 nm) versus bonded to R through boronic ester bonds (λmax = 491 nm), Fig. S3.† To see if this change in absorbance could potentially be used to detect diol product 3 in a crude aldol reaction mixture, we performed control experiments titrating each aldol reaction component into a solution of PV and R. The change in absorbance at 520 nm was monitored, and as shown in Fig. 1, a sample of the purified diol product 3 resulted in an observable spectroscopic change whereas a smaller degree of dye displacement was observed for the addition of either starting material. These controls also led us to hypothesize that the assay might err on the side of overestimating reaction yield, since some of the observed displacement in a reaction mixture would be due to the remaining excess of 1 (three equivalents present initially) in addition to any diol product 3 formed. Controls to probe the dye displacement by the peptide catalysts, 4a–e, were not necessary because the catalyst is not present during the assay since it remains resin-bound (Scheme 1b). The dissociation constant between the diol product 3 and R was calculated to be 1.83 mM.§
To test the ability of the dye-displacement assay to evaluate catalytic peptides, we investigated whether the assay could detect 3 in a crude reaction mixture using interpolation from a standard curve to determine the concentration of diol product and thus conversion of the reaction. The assay-calculated conversion was compared to the crude 1H NMR conversion for five catalytic species, which included a known catalytic sequence, three derivatives of this sequence with unknown catalytic properties, and unfunctionalized Wang resin, 4e, to serve as a negative control. The known catalytic sequence used was VDPGL (4a), which was reported as a primary amine aldolase-mimetic aldol catalyst by the Da group,10 with the high catalytic activity and enantioselectivity attributed to the β-turn conformation conferred by the DPro-Gly residues.9,12,32 In the report of this sequence, the peptide was used in solution with additional additives, therefore we expected a modulation in catalytic ability under our reaction conditions; however, there is precedent for the use of resin-supported peptides as catalysts.33,34 We also synthesized variants of this catalyst with different residues in the turn positions, VPGL (4b) and VDPPL (4c), and one sequence with residues unlikely to nucleate a β-turn, VAAL (4d), which had unknown, but presumably lower, catalytic activity than VDPGL. Therefore we sought to determine if the assay could differentiate between high, intermediate, and low yielding catalysts (4a, 4d, and 4e, respectively) and to see to which of these categories 4b and 4c belonged.
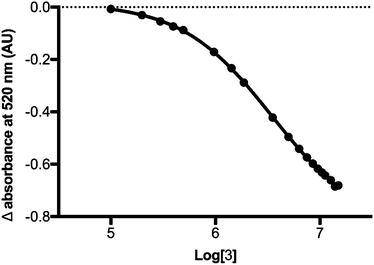
For the peptide-catalysed reactions, 2 was incubated with three equivalents of 1 and 0.2 equivalents peptide catalyst 4 in dichloromethane for 48 h at room temperature with gentle agitation. The crude reaction mixtures were separated by filtration from the solid-supported catalysts and the conversion was calculated by the colorimetric displacement assay, as well as by 1H NMR spectroscopy as an independent means of verifying the reaction yield. The assay was performed by adding a single aliquot of crude reaction mixture to a solution of R and PV and monitoring the change in absorbance of the R·PV solution at 520 nm. Using interpolation from a standard curve relating change in absorbance to concentration of diol product 3 (Fig. 2), the yield of diol product in each crude reaction mixture was calculated.
A comparison of the conversion as calculated by the assay and by 1H NMR spectroscopy is shown in Fig. 3. As a general trend, the calculated conversions correlate, and it is clear that the assay can be used to distinguish between non-catalytic species (4e), intermediate (4d), and highly active catalysts (4a–c). However, the results – specifically the variance between the assay and NMR conversion, and the assay-calculated conversion above 100% for 4b – indicate that the dye displacement measure is more appropriate as a qualitative tool to identify lead catalytic sequences rather than a quantification method. As expected, the direction of error was toward an overestimation of reaction conversion by the assay, likely due to some dye displacement by the starting materials. A comparison of parent sequence VDPGL (4a) to derivatives containing different turn-nucleating residues VPGL (4b) and VDPPL (4c) highlights the importance of studying analogues of lead catalytic sequences, as small changes to the peptide structure, such as varying the stereochemistry at a single site (VDPGL vs. VPGL), resulted in a significant increase in catalytic activity relative to the parent compound.¶
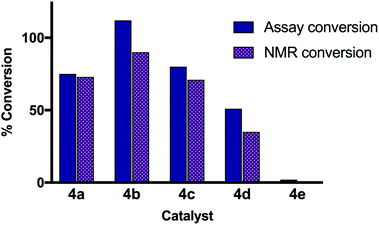 | ||
| Fig. 3 The reaction converstion for five catalytic species was calculated by both colorimetric displacement assay (solid blue bars) and crude 1H NMR (textured purple bars). | ||
We also tested if the assay would work for other aldol reaction substrates. It is expected that most vicinal diol-containing analytes would result in dye displacement, and in our examination of several other aldehyde substrates we found that the assay-calculated conversion correlated with the reaction conversion calculated by crude 1H NMR (Fig. S12 in the ESI†). The dye displacement assay may be a useful way to identify catalysts for specific substrates of interest.
In conclusion, we have shown that competitive colorimetric displacement can be used as a qualitative method for the rapid evaluation of catalytic peptides. We validated the assay using a comparison to the more traditional method of 1H NMR spectroscopy, and from a small peptide library we identified a novel sequence with improved catalytic activity relative to a previously reported catalyst. The ability to use visible light absorption for catalyst evaluation will enable the screening of larger peptide libraries to identify highly active novel catalytic sequences. The assay we have developed is not limited to the evaluation of peptides and could be used to study other types of catalysts such as organocatalysts or enzymes.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors gratefully acknowledge Professor J. Wollack and the use of the LCQ Fleet LCMS at Saint Catherine University, as well as helpful discussions with Professor D. Cao. A. M. M. G. was supported by an ACS DOC SURF award. This research has been funded in part by a grant to Macalester College from the Howard Hughes Medical Institute through the Precollege and Undergraduate Science Education Program, as well as through the Macalester College Leonard, Keck, and MacKnight Haan Ludwig Summer Research Funds.Notes and references
- E. A. C. Davie, S. M. Mennen, Y. Xu and S. J. Miller, Chem. Rev., 2007, 107, 5759 CrossRef PubMed.
- H. Wennemers, Chem. Commun., 2011, 47, 12036 RSC.
- B. Lewandowski and H. Wennemers, Curr. Opin. Chem. Biol., 2014, 22, 40 CrossRef CAS PubMed.
- K. Akagawa, R. Suzuki and K. Kudo, Asian J. Org. Chem., 2014, 3, 514 CrossRef CAS.
- B. Schetter and R. Mahrwald, Angew. Chem., Int. Ed., 2006, 45, 7506 CrossRef CAS PubMed.
- A. Córdova, W. Notz and C. F. Barbas, Chem. Commun., 2002, 3024 RSC.
- B. List, R. A. Lerner and C. F. Barbas, J. Am. Chem. Soc., 2000, 122, 2395 CrossRef CAS.
- P. Krattiger, R. Kovasy, J. D. Revell, S. Ivan and H. Wennemers, Org. Lett., 2005, 7, 1101 CrossRef CAS PubMed.
- J. D. Revell, D. Gantenbein, P. Krattiger and H. Wennemers, Pept. Sci., 2006, 84, 105 CrossRef CAS PubMed.
- F.-C. Wu, C.-S. Da, Z.-X. Du, Q.-P. Guo, W.-P. Li, L. Yi, Y.-N. Jia and X. Ma, J. Org. Chem., 2009, 74, 4812 CrossRef CAS PubMed.
- W. Zou, I. Ibrahem, P. Dziedzic, H. Sundén and A. Córdova, Chem. Commun., 2005, 4946 RSC.
- K. Akagawa, S. Sakamoto and K. Kudo, Tetrahedron Lett., 2005, 46, 8185 CrossRef CAS.
- S. Bayat, B. A. Tejo, E. Abdulmalek, A. B. Salleh, Y. M. Normi and M. B. Abdul Rahman, RSC Adv., 2014, 4, 38859 RSC.
- A. J. Metrano, N. C. Abascal, B. Q. Mercado, E. K. Paulson and S. J. Miller, Chem. Commun., 2016, 52, 4816 RSC.
- A. J. Metrano, N. C. Abascal, B. Q. Mercado, E. K. Paulson, A. E. Hurtley and S. J. Miller, J. Am. Chem. Soc., 2017, 139, 492 CrossRef CAS PubMed.
- M. Wiesner, M. Neuburger and H. Wennemers, Chem. – Eur. J., 2009, 15, 10103 CrossRef CAS PubMed.
- J. D. Revell and H. Wennemers, Adv. Synth. Catal., 2008, 350, 1046 CrossRef CAS.
- J. D. Revell and H. Wennemers, Curr. Opin. Chem. Biol., 2007, 11, 269 CrossRef CAS PubMed.
- K. Akagawa, N. Sakai and K. Kudo, Angew. Chem., Int. Ed., 2015, 54, 1822 CrossRef CAS PubMed.
- K. Akagawa, J. Satou and K. Kudo, J. Org. Chem., 2016, 81, 9396 CrossRef CAS PubMed.
- M. W. Giuliano, C.-Y. Lin, D. K. Romney, S. J. Miller and E. V. Anslyn, Adv. Synth. Catal., 2015, 357, 2301 CrossRef CAS PubMed.
- P. A. Lichtor and S. J. Miller, ACS Comb. Sci., 2011, 13, 321 CrossRef CAS PubMed.
- G. T. Copeland and S. J. Miller, J. Am. Chem. Soc., 1999, 121, 4306 CrossRef CAS.
- P. Krattiger, C. McCarthy, A. Pfaltz and H. Wennemers, Angew. Chem., Int. Ed., 2003, 42, 1722 CrossRef CAS PubMed.
- I. Lingard, G. Bhalay and M. Bradley, Chem. Commun., 2003, 2310 RSC.
- B. T. Nguyen and E. V. Anslyn, Coord. Chem. Rev., 2006, 250, 3118 CrossRef CAS.
- L. You, D. Zha and E. V. Anslyn, Chem. Rev., 2015, 115, 7840 CrossRef CAS PubMed.
- E. V. Anslyn, J. Org. Chem., 2007, 72, 687 CrossRef CAS PubMed.
- S. H. Shabbir, C. J. Regan and E. V. Anslyn, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 10487 CrossRef CAS PubMed.
- L. Zhu and E. V. Anslyn, J. Am. Chem. Soc., 2004, 126, 3676 CrossRef CAS PubMed.
- L. Zhu, Z. Zhong and E. V. Anslyn, J. Am. Chem. Soc., 2005, 127, 4260 CrossRef CAS PubMed.
- T. Tanaka, K. Akagawa, M. Mitsuda and K. Kudo, Adv. Synth. Catal., 2013, 355, 294 CAS.
- Y. Arakawa and H. Wennemers, ChemSusChem, 2013, 6, 242 CrossRef CAS PubMed.
- Y. Arakawa, M. Wiesner and H. Wennemers, Adv. Synth. Catal., 2011, 353, 1201 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR and MS spectra. See DOI: 10.1039/c7ob02032e |
| ‡ Calculated using GraphPad Prism 7 using the “Receptor binding: saturation binding — one site specific binding” function. |
| § Calculated using GraphPad Prism 7 using the “Receptor binding: competitive binding — one site – Fit Ki” function. |
| ¶ Although the yields for the parent peptide catalyst 4a and the more active catalyst 4b were high under our reaction conditions, the stereoselectivity was low (data in ESI†). This may be due to the difference between our reaction conditions and the originally reported reaction conditions for 4a. |
| This journal is © The Royal Society of Chemistry 2017 |