Direct carboxamidation of cyclic 2-diazo-1,3-diketones by Rh2(OAc)4-catalyzed isocyanide insertion–hydrolysis†
Abstract
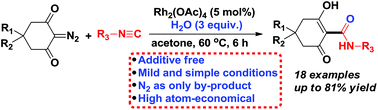
A novel and efficient strategy for the synthesis of 2-hydroxy-6-oxocyclohex-1-enecarboxamides through a Rh2(OAc)4-catalyzed direct carboxamidation of cyclic 2-diazo-1,3-diketones has been developed. The method features readily available starting materials, easy scalability, mild reaction conditions and a simple work-up for product isolation, which makes this strategy highly attractive. A tentative mechanism involving an isocyanide insertion and hydrolysis sequence for this reaction is proposed.


 Please wait while we load your content...
Please wait while we load your content...