Iodine-catalyzed oxidative multiple C–H bond functionalization of isoquinolines with methylarenes: an efficient synthesis of isoquinoline-1,3,4(2H)-triones†
Abstract
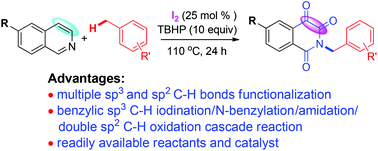
An iodine-catalyzed multiple C–H bond functionalization of isoquinolines with methylarenes via a successive benzylic sp3 C–H iodination/N-benzylation/amidation/double sp2 C–H oxidation sequence is developed. This reaction utilizes un-functionalized isoquinolines and readily available methylarenes as starting materials, proceeds under metal-free conditions, and avoids a multi-step experimental operation, to make it an efficient and practical method for the synthesis of N-benzyl isoquinoline-1,3,4-triones.


 Please wait while we load your content...
Please wait while we load your content...