Asymmetric synthesis of isoquinolinonaphthyridines catalyzed by a chiral Brønsted acid†
Abstract
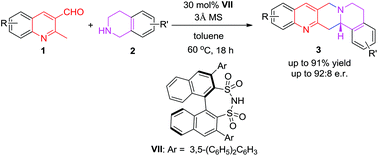
A catalytic asymmetric method for the synthesis of chiral isoquinolinonaphthyridines has been developed. A chiral disulfonimide catalyzes a redox cyclization reaction between 2-methyl-3-aldehydeazaarenes and 1,2,3,4-tetrahydroisoquinolines to deliver a range of isoquinolinonaphthyridines with good to high yields (up to 91%) and up to 92 : 8 er.


 Please wait while we load your content...
Please wait while we load your content...