Copper-catalyzed, stereoconvergent, cis-diastereoselective borylative cyclization of ω-mesylate-α,β-unsaturated esters and ketones†
Abstract
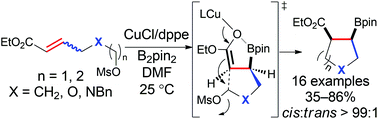
The Cu(I)-catalyzed stereoconvergent borylative cyclization of ω-mesylate-α,β-unsaturated compounds is facilitated by a simple Cu-bisphosphine catalyst. This reaction provides a novel route to cis-β-boron-substituted five- and six-membered carbocycle and heterocycle esters. Mechanistic studies indicate that stereoconvergence and cis-substitution likely stem from the rapid enolation of the borylcopper adduct with the substrate double bond and the formation of a five-membered intermediate, respectively.


 Please wait while we load your content...
Please wait while we load your content...