Selective turn-on fluorescence sensing of sulfate in aqueous–organic mixtures by an uncharged bis(diamidocarbazole) receptor†
Abstract
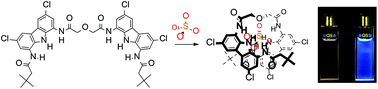
A linear, uncharged, hydrogen bonding receptor A with two carbazole-based binding domains was synthesised and evaluated for its anion binding properties in DMSO/H2O mixtures. 1H NMR titrations revealed that, in DMSO/H2O 0.5%, A forms both 1 : 1 and 1 : 2 complexes with SO42−, H2PO4−, PhCOO− and Cl−. In 1 : 1 complexes the receptor encloses the tetrahedral anions tightly, forming a helical structure, while Cl− binds with a single carbazole unit only. In the presence of 10% of water the 1 : 2 complexes with SO42− and PhCOO− disappear, and the respective 1 : 1 binding constants decrease sufficiently to be quantified by UV-Vis titration. In this highly competitive medium, A binds sulfate with K1:1 = 105.47 M−1, i.e., it binds approx. 30, 360 and >1000 times more strongly than H2PO4−, PhCOO− and Cl−, respectively. Furthermore, the association with sulfate is over 50 times stronger than that for a model diamidocarbazole 1 under identical conditions, suggesting a very strong chelating effect due to the diglycoyl linker. Increasing the amount of water to 25% (the solubility limit of A) lowers the 1 : 1 binding constant with SO42− to 103.73 M−1. Receptor A was shown to act as a selective turn-on fluorescent sensor for sulfate, able to sense sulfate in sulfate-rich mineral water.


 Please wait while we load your content...
Please wait while we load your content...