Synthesis, duplex-forming ability, enzymatic stability, and in vitro antisense potency of oligonucleotides including 2′-C,4′-C-ethyleneoxy-bridged thymidine derivatives†
Abstract
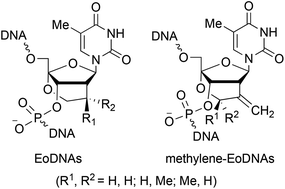
We synthesized thymidine derivatives of 2′-C,4′-C-ethyleneoxy-bridged 2′-deoxyribonucleic acids with an 8′-methyl group ((R)-Me-EoDNA and (S)-Me-EoDNA) and without any substituent (EoDNA). Oligonucleotides including these EoDNAs showed high hybridization abilities with complementary RNA and excellent enzymatic stabilities compared with natural DNA. Moreover, the in vitro antisense potency of oligonucleotides with these EoDNAs and our recently reported methylene-EoDNAs was investigated and compared with that of LNA, which is a practical chemical modification for oligonucleotide-therapeutic agents. The results showed that EoDNAs and methylene-EoDNAs could be promising candidates for antisense technology.


 Please wait while we load your content...
Please wait while we load your content...