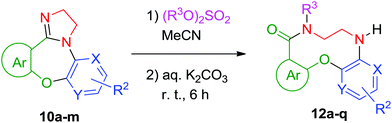Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structurally diverse arene-fused ten-membered lactams accessed via hydrolytic imidazoline ring expansion†
Alexander
Sapegin
,
Angelina
Osipyan
and
Mikhail
Krasavin
 *
*
Institute of Chemistry, Saint Petersburg State University, 26 Universitetskii prospekt, Peterhof 198504, Russia. E-mail: m.krasavin@spbu.ru
First published on 15th March 2017
Abstract
Imidazoline-fused [1,4]oxazepines (prepared in two simple steps from methyl 2-hydroxyaroates, ethylene diamine and bis-electrophilic aromatics) undergo a facile, good-yielding hydrolytic imidazoline ring expansion (HIRE) upon N-alkylation and treatment with aqueous K2CO3. The resulting arene-fused ten-membered lactams significantly add to the contemporary arsenal of small-molecule scaffolds where medium-sized ring systems are severely underrepresented.
Small molecule libraries containing medium-sized (8- to 11-membered) rings are currently recognized as a drug discovery platform with many advantages compared to linear scaffolds or those based on flat heteroaromatic rings.1 The introduction of a cycle drastically reduces the rotatable bond count (compared to a linear molecule), which improves permeability through cell membranes and intestinal absorption.2 Yet medium-sized rings are more flexible compared to their smaller-size counterparts and thus can adopt a greater number of conformations, amongst which the one with an optimum presentation of the molecular periphery toward the biotarget has a higher chance to be found.3 This particular consideration has made medium-sized cyclic scaffolds popular, among other areas, in drug discovery against poorly ‘druggable’ targets such as protein–protein interactions.4
The ring closure approaches to medium-sized ring construction are encumbered by entropy constraints,5 hence ring expansion has manifested itself as a complementary and a lot more prolific strategy.6 The latter usually involves the construction of a polycyclic system with some degree of instability where breaking one of the central bonds leads to stabilization (e.g., via aromatization or strain relief) and thus provides the driving force for ring expansion. Such an approach is eloquently illustrated, among many others, by the recent reports from the Tan group describing oxidative ring expansion of cyclic enol ethers (or enamines) 1 to give macrocyclic lactones (or lactams) 2,7 or re-aromatization of tricyclic quinone precursors 3 generating ring-expanded products 4.8
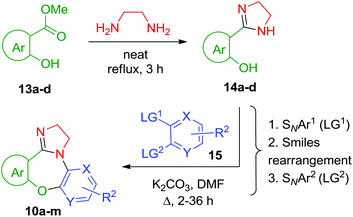
Our recent interest in N-(hetero)aryl 2-imidazolines for drug discovery9 and scaffold-oriented synthesis10 alarmed us due to the fact that polycyclic 2-imidazolines 5 have not been explored in the context of ring expansion (though such a possibility is indicated by the expansion of related polycyclic systems11 and benzimidazoles12 on treatment with aluminium hydride agents reported in the literature). When activated by protonation13 or alkylation14 to form 6, 2-imidazolines can undergo hydrolysis via tetrahedral intermediate 7. We reasoned that the latter can potentially evolve in two alternative directions one of which could lead to 2-aminoethyl side chain expulsion (to give 8) and the other to the formation of ring-expanded product 9. For monocyclic 2-imidazolines, forms 8 and 9 were shown to be interconvertible depending on the pH.15 Bicyclic 2-imidazoline-containing compounds such as 5 (i.e. with N1 and C2 at the bridgehead) are relatively scarce, which may explain why ring-expansion methodologies involving hydrolytic disruption of a 2-imidazoline moiety are lacking in the literature (though such a possibility was indicated by an isolated example related to this study).16 Recently, we gained a practically simple access to tetracyclic [1,4]oxazepines 10,17 which, in our view, represent suitable objects for enabling the hydrolytic imidazoline ring expansion (HIRE) (Fig. 1). Herein, we report on a successful realization of this potential in compounds 10.
 | ||
| Fig. 1 Recent examples of ring expansion toward medium-sized rings and the goal of the present study. | ||
In order to verify, in principle, the possibility of engaging compounds 10 in a ring expansion sequence 5 → 6 → 8, we identified a suitable 2-imidazoline quaternization protocol by exposing previously reported tetracyclic compound 10a17 (prepared as detailed below) to various N-methylation conditions (Table 1). The employment of dimethyl sulfate in acetonitrile at ambient temperature (entry 7) gave the best isolated yield of respective imidazolinium salt 11a. Surprisingly, all attempts to bring about the same transformation using either MeI or MeOTs left the starting material unchanged.
For the hydrolytic ring expansion (such as 6 → 8) envisioned as the likely fate for compound 10a we tested various conditions employing diluted base solutions in pure water or aqueous organic solvents (Table 2). To our utmost delight, the ring expansion did take place in all cases tested while the best isolated yield of ten-membered lactam 12a was obtained on exposing 11a to 0.1% w/v K2CO3 solution in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeCN–water over 6 hours at ambient temperature (entry 6). Employing no base (entry 10) resulted in no conversion.
1 MeCN–water over 6 hours at ambient temperature (entry 6). Employing no base (entry 10) resulted in no conversion.
| Entry | Base | Solvent | Time, h | Isolated yield (%) |
|---|---|---|---|---|
| a Reaction was performed at 40 °C. | ||||
| 1 | NaOH (5%) | H2O | 5 | 34 |
| 2 | K2CO3 (5%) | H2O | 8 | 51 |
| 3 | NH4OH (5%) | H2O | 8 | 48 |
| 4 | K2CO3 (1%) | H2O | 8 | 63 |
| 5 | K2CO3 (0.1%) | H2O | 8 | 74 |
| 6 | K2CO3 (0.1%) | H2O/MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
6 | 79 |
| 7 | K2CO3 (0.1%) | H2O/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
6 | 71 |
| 8 | K2CO3 (0.1%) | H2O/THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
6 | 75 |
| 9 | K2CO3 (0.1%) | H2O/MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
5a | 77 |
| 10 | None | H2O/MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
24 | 0 |
Besides the 1H and 13C NMR and high-resolution mass-spectrometry data, the structure of 12a was confirmed by single-crystal X-ray analysis (ESI†). We also performed the transformation of 10a to 12a without the interim isolation of 11a (by adding an equal volume of 0.2% aqueous K2CO3 solution to the reaction mixture after quaternization of 10a was complete) and found the resulting yield (73%) to be significantly higher than the combined yield (58%) over separate quanternization/ring expansion steps (vide supra). Therefore, the one-pot protocol was adopted for all ring expansion examples discussed below.
Tetracyclic imidazoline-fused [1,4]oxazepines 10a–m were prepared in two practically simple steps from 2-hydroxyaroate esters 13 and ethylene diamine followed by coupling of resulting 2-hydroxyarylimidazolines 14 with bis-electrophilic (hetero)aromatic substrates 15 as described previously (specific structures of cyclocondensation partners 14–15 can be found in the ESI†).17 As was also demonstrated earlier,17,18 the regiochemistry of compounds 10a–m is defined by two SNAr events leading to the [1,4]oxazepine cycle formation being intermitted by a Smiles rearrangement (Scheme 1).
Gratifyingly, the N-alkylation/HIRE protocol developed for 10a (vide supra) was found to be fully transferrable (Scheme 2) onto the other twelve substrates (10b–m). Surprisingly, N-alkylation with diethyl sulfate required refluxing temperatures, which resulted in generally lower yields of [1,4,7]oxadiazecin-9(6H)-ones (16n–q). Notably, compounds 16a–q (Fig. 2) represent three distinctly novel19 scaffolds: 7,8-dihydro-5H-benzo[i]pyrido[3,2-b][1,4,7]oxadiazecin-9(6H)-one (16a–c, 16g, 16j–o, 16q), 7,8-dihydro-5H-dibenzo[b,i][1,4,7]oxadiazecin-9(6H)-one (16e–f, 16h, 16p), and 7,8-dihydro-5H-benzo[i]pyrazino[2,3-b][1,4,7]oxadiazecin-9(6H)-one (16d and 16i). The medium to good isolated yields of compounds 16a–q shown in Fig. 2 were obtained using the same general procedure (ESI†) and have not been optimized for specific compounds. The nature of substituents in the salicylate portion of the compounds (shown in green color) does not appear to have any influence on the product yields. The electron-poor character of the other aromatic portion of the molecule (shown in blue color) is mandated by the method used to prepare precursors 10a–m (vide infra). However, it does not appear to have a significant impact on the outcome of the reaction.
In conclusion, we have reported the first example where hydrolytic instability of the 2-imidazoline moiety (activated by N-alkylation) is exploited to achieve ring expansion of a smaller cycle into a medium-ring system. The starting materials are distinctly easy to prepare and the ring expansion is brought about by two chemical operations performed in a one-pot format. Considering the scarcity of medium-sized scaffolds in the contemporary arsenal of small molecules for biological screening, the approach described is expected to significantly aid in filling this void. Research aimed at expanding the diversity of polycyclic systems as substrates for HIRE is underway in our laboratories; we will report the results in due course.
This research was supported by the Russian Scientific Fund (project grant 14-50-00069). We thank the Research Centre for Magnetic Resonance, the Centers for Chemical Analysis and Materials Research and the Centre for X-ray Diffraction Methods of Saint Petersburg State University Research Park for the analytical data.
Notes and references
- L. G. Baud, M. A. Manning, H. L. Arkless, T. C. Stephens and W. P. Unsworth, Chem. – Eur. J., 2017, 23, 2225 CrossRef CAS PubMed.
- D. F. Veber, S. R. Johnson, H.-Y. Cheng, B. R. Smith, K. W. Ward and K. D. Kopple, J. Med. Chem., 2002, 45, 2615 CrossRef CAS PubMed.
- L. A. Marcaurelle, E. Comer, S. Dandapani, J. R. Duvall, B. Gerard, S. Kesavan, M. D. Lee, H. Liu, J. T. Lowe, J.-C. Marie, C. A. Mulrooney, B. A. Pandya, A. Rowley, T. D. Ryba, B.-C. Suh, J. Wei, D. W. Young, L. B. Akella, N. T. Ross, Y. L. Zhang, D. M. Fass, S. A. Reis, W.-N. Zhao, S. J. Haggarty, M. Palmer and M. A. Foley, J. Am. Chem. Soc., 2010, 132, 16962 CrossRef PubMed.
- J. Kim, J. Jung, J. Koo, W. Cho, W. S. Lee, C. Kim, W. Park and S. B. Park, Nat. Commun., 2016, 7, 13196 CrossRef CAS PubMed.
- I. Shiina, Chem. Rev., 2007, 107, 239 CrossRef CAS PubMed.
- (a) L. Huang, L. X. Dai and S. L. You, J. Am. Chem. Soc., 2016, 138, 5793 CrossRef CAS PubMed; (b) J. E. Hall, J. V. Matlock, J. W. Ward and J. Clayden, Angew. Chem., Int. Ed., 2016, 55, 11153 CrossRef CAS PubMed.
- F. Kopp, C. F. Stratton, L. B. Akella and D. S. Tan, Nat. Chem. Biol., 2012, 8, 358 CrossRef CAS PubMed.
- R. A. Bauer, T. A. Wenderski and D. S. Tan, Nat. Chem. Biol., 2013, 9, 21 CrossRef CAS PubMed.
- (a) P. Sarnpitak, P. Mujumdar, C. Morisseau, S. H. Hwang, B. Hammock, V. Iurchenko, S. Zozulya, A. Gavalas, A. Geronikaki, Y. Ivanenkov and M. Krasavin, Eur. J. Med. Chem., 2014, 84, 160 CrossRef CAS PubMed; (b) M. Krasavin, P. Mujumdar, V. Parchinsky, T. Vinogradova, O. Manicheva and M. Dogonadze, J. Enzyme Inhib. Med. Chem., 2016, 31, 1146 CrossRef CAS PubMed; (c) C. T. Supuran, S. Kalinin, M. Tanç, P. Sarnpitak, P. Mujumdar, S.-A. Poulsen and M. Krasavin, J. Enzyme Inhib. Med. Chem., 2016, 31(Suppl. 1), 197 CrossRef CAS PubMed; (d) P. Sarnpitak, P. Mujumdar, A. Shetnev, M. Dorogov and M. Krasavin, Tetrahedron Lett., 2015, 56, 2827 CrossRef; (e) P. Sarnpitak, P. Mujumdar, P. Taylor, M. Cross, M. J. Coster, A.-D. Gorse, M. Krasavin and A. Hofmann, Biotechnol. Adv., 2015, 33, 941 CrossRef CAS PubMed.
- (a) M. Krasavin, Tetrahedron Lett., 2012, 53, 2876 CrossRef CAS; (b) P. Mujumdar, M. Korsakov, M. Dorogov and M. Krasavin, Synlett, 2014, 2323 CAS; (c) D. Dar'in and M. Krasavin, J. Org. Chem., 2016, 81, 12514 CrossRef PubMed.
- H. Yamamoto and K. Maruoka, J. Am. Chem. Soc., 1981, 103, 4186 CrossRef CAS.
- T. Shvidenko, K. Nazarenko, K. Shvidenko and A. Kostyuk, Tetrahedron Lett., 2014, 55, 279 CrossRef CAS.
- P. Mujumdar, T. Grkovic and M. Krasavin, Tetrahedron Lett., 2013, 54, 3336 CrossRef CAS.
- C. Xia, J. Hao, Y. Tang, Y. Ni and P. Zhou, Synth. Commun., 2002, 32, 1457 CrossRef CAS.
- B. M. Fernandez, A. M. Reverdito, G. A. Paolucci and I. A. Perillo, J. Heterocycl. Chem., 1987, 24, 1717 CrossRef.
- J. M. Villalgordo and H. Heimgartner, Helv. Chim. Acta, 1997, 80, 748 CrossRef CAS.
- K. Karamysheva, E. Reutskaya, A. Sapegin, M. Dorogov and M. Krasavin, Tetrahedron Lett., 2015, 56, 5632 CrossRef CAS.
- (a) A. V. Sapegin, S. A. Kalinin, A. V. Smirnov, M. V. Dorogov and M. Krasavin, Synthesis, 2012, 2401 CAS; (b) A. V. Sapegin, S. A. Kalinin, A. V. Smirnov, M. V. Dorogov and M. Krasavin, Tetrahedron, 2014, 70, 1077 CrossRef CAS; (c) A. V. Sapegin, S. A. Kalinin, A. V. Smirnov, M. V. Dorogov and M. Krasavin, Eur. J. Org. Chem., 2015, 1333 CrossRef CAS; (d) A. Sapegin, E. Reutskaya, A. V. Smirnov, M. Korsakov and M. Krasavin, Tetrahedron Lett., 2016, 57, 5877 CrossRef CAS; (e) A. Sapegin, V. Panova, E. Reutskaya, A. V. Smirnov and M. Krasavin, Tetrahedron, 2016, 72, 7570 CrossRef CAS.
- According to SciFinder® search performed on February 10th, 2017.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data, copies of the NMR spectra. CCDC 1520950. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ob00535k |
| This journal is © The Royal Society of Chemistry 2017 |