Devender Mandala, Sravanthi Chada and Paul Watts
Org. Biomol. Chem., 2017,15, 3444-3454
DOI:
10.1039/C7OB00480J,
Paper
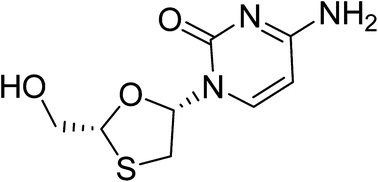
We report the first continuous flow synthesis of lamivudine, an antiretroviral drug used in the treatment of HIV/AIDS and hepatitis B. The key intermediate (5-acetoxy oxathiolane) was prepared by an integrated two step continuous flow process from L-menthyl glyoxalate hydrate in a single solvent, in 95% overall conversion. For the crucial glycosidation reaction, using pyridinium triflate as the novel catalyst, an improved conversion of 95% was obtained. The overall isolated yield of the desired isomer of lamivudine (40%) was improved in the flow synthesis compared to the batch process.