Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Acid-promoted cyclization of 2,4-diaryl-1,1,1-trifluorobut-3-en-2-oles and their TMS-ethers into CF3-indenes†
Matvei Yu.
Martynov
a,
Roman O.
Iakovenko
a,
Anna N.
Kazakova
a,
Irina A.
Boyarskaya
a and
Aleksander V.
Vasilyev
 *ab
*ab
aInstitute of Chemistry, Saint Petersburg State University, Universitetskaya nab., 7/9, Saint Petersburg, 199034, Russia
bDepartment of Chemistry, Saint Petersburg State Forest Technical University, Institutsky per., 5, Saint Petersburg, 194021, Russia. E-mail: aleksvasil@mail.ru
First published on 27th February 2017
Abstract
2,4-Diaryl-1,1,1-trifluorobut-3-en-2-oles and their TMS-ethers in H2SO4 at room temperature in just 2 min are quantitatively cyclized into 1-aryl-3-trifluoromethyl-1H-indenes. The reaction proceeds through an intermediate formation of the corresponding CF3-allyl cations, which are cyclized regioselectively at the allyl carbon atom most remote from the CF3-group. The obtained CF3-indenes in solution of EtOAc in the presence of silica gel at room temperature over 4 h are quantitatively isomerized into 3-aryl-1-trifluoromethyl-1H-indenes.
Introduction
Allyl alcohols are valuable synthons in organic chemistry. Recently we have shown1–3 that reactions of some trifluoromethyl substituted allyl alcohols with arenes under the action of Brønsted or Lewis acids have resulted in the formation of trifluoromethylated alkenes, indanes, or indenes. Synthesis of indenes4–6 is an actual goal in chemistry, biology, and medicine. For instance, indene derivatives are widely used as biologically active compounds,7,8 and ligands for metallo-complexes.9–12 Introduction of a trifluoromethyl group into the indene core may confer new chemical, biological (lipophilicity and bioavailability), and physical properties to the molecules, due to the strong electron acceptor characteristic of the CF3 group. CF3-indenes are rather rare compounds, and there are just a few reports on their synthesis.13–17 For instance, Langlois et al. showed just one example of BF3-promoted cyclization of CF3-allyl alcohol into the corresponding CF3-indene.13The main goal of this work was a study of acid-promoted electrophilic transformation of 2,4-diaryl-1,1,1-trifluorobut-3-en-2-oles 2 and their TMS-ethers 1. CF3-TMS-ethers 1 are easily available from chalcones by trifluoromethylation of the carbonyl group with CF3TMS followed by desilylation with SnCl2 or aqueous HCl leading to CF3-allyl alcohols 2 (Scheme 1).
 | ||
| Scheme 1 Synthesis of CF3-TMS-ethers 1 and the corresponding CF3-alcohols 2 from chalcones (see substituents R1, R2 in aryl rings Ar1, Ar2, respectively, in Table 1). | ||
Results and discussion
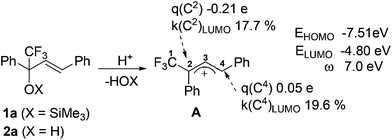
First, we decided to study plausible reaction cationic intermediates by means of quantum chemical calculations. One would expect that compounds 1/2 under the action of Brønsted or Lewis acids could give rise to the corresponding CF3-allyl cations.13 To estimate electronic characteristics of these cations we carried out DFT calculations of species A generated from 1a/2a by the protonation of the OX group (X = TMS or H), followed by elimination of HOX (Fig. 1). The following parameters were calculated: E – energy of the HOMO and LUMO, global electrophilicity index ω,18,19 natural charges q, contribution of the atomic orbital to the molecular orbital k. The calculation shows that carbon C2 bears a large negative charge −0.21e, but carbon C4 has a small positive charge and gives rather big contribution (19.6%) to the LUMO. These data demonstrate that the reactive electrophilic center of cation A should be carbon C4 by both charge and orbital control. Species A possesses a big value of ω index 7.0 eV, pointing out its high electrophilicity.Then we carried out reactions of the series of compounds 1/2 under the action of various acidic reagents (Table 1). Indeed, the cyclization of 1/2 into CF3-indenes 3 takes place showing that carbon C4 is a reactive center in the corresponding intermediate cations A. Among all other tested Brønsted and Lewis acids, sulfuric acid (H2SO4) was found to be one of the best for this transformation, and the reaction in this acid took just 2 min (Table 1). Alcohol 2a was not converted into acetic acid (room temperature, 4 h), and remained unreacted. On the other hand, strong Lewis acids AlX3 (X = Cl, Br) in the reaction with 2a led to complex oligomeric mixtures. The same reaction in trifluoroacetic needed a longer time (room temperature, 1 h) and gave 3a in 88% yield. Triflic acid CF3SO3H (TfOH) may be used as well; indene 3a was formed in 2–3 min in 82% yield in this acid. However, we finally chose H2SO4 because it is a cheap and easy to handle reagent.
| Entry | Starting compounds | Reaction products | |||
|---|---|---|---|---|---|
| 1/2 | R1, R2 in 1/2, R2 in 3 | R1 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (yield, %) a (yield, %) |
||
| R1 | R2 | ||||
a Isolated yields.
b Yield obtained with the reaction in CF3CO2H (50 equiv.), instead of H2SO4, for 5 min.
c Two regioisomers 3j1 and 3j2 with a ratio 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 96% yield were obtained.
d The mixture of isomeric indenes 3q and 4q in a ratio of 8 1 and 96% yield were obtained.
d The mixture of isomeric indenes 3q and 4q in a ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, was obtained (see Scheme 2). 1, respectively, was obtained (see Scheme 2).
|
|||||
| 1 | 1a/2a | H | H | H | 3a (90) |
| 2 | 1b/2b | 4-Me | H | 6-Me | 3b (99) |
| 3 | 1c/2c | H | 4-Me | H | 3c (99) |
| 4 | 1d/2d | 4-Me | 4-Me | 6-Me | 3d (99) |
| 5 | 1e/2e | 4-MeO | H | 6-MeO | 3e (97) (97b) |
| 6 | 1f/2f | H | 4-MeO | H | 3f (97) (97b) |
| 7 | 1g/2g | 3,4-Di(MeO) | H | 5,6-Di(MeO) | 3g (95) (98b) |
| 8 | 1h/2h | 4-MeO | 4-MeO | 6-MeO | 3h (10) (92b) |
| 9 | 1i/2i | 4-Me | 3,4-Di(MeO) | 6-Me | 3i (20) (50b) |
| 10c | 1j/2j | 3,4-Di(Me) | H | 5,6-Di(Me) | 3j1 (53) |
| 6,7-Di(Me) | 3j2 (43) | ||||
| 11 | 1k | 2,4-Di(Me) | H | 4,6-Di(Me) | 3k (97) |
| 12 | 1l | 2,5-Di(Me) | H | 4,7-Di(Me) | 3l (97) |
| 13 | 1m/2m | 2,4,6-Tri(Me) | H | 4,6,7-Tri(Me) | 3m (97) |
| 14 | 1n/2n | H | 3,4-OCH2O | H | 3n (68) (91b) |
| 15 | 1o/2o | H | 4-Cl | H | 3o (97) |
| 16 | 1p | 4-F | 3,4-Di(Me) | 6-F | 3p (97) |
| 17 | 1q | 4-F | 3,4-Di(MeO) | 6-F | 3q (74)d |
Both CF3-TMS-ethers 1 and CF3-allyl alcohols 2 gave the same indenes with the same high yields (Table 1). In most of the cases, the formation of indenes 3a–q was quantitative (Table 1). However, in H2SO4 compounds 3h and 3i bearing donating methoxy groups were formed in low yields of 10 and 20%, respectively, due to the consequent transformations of these electron-rich indenes in H2SO4. The use of less acidic CF3CO2H resulted in much higher yields of 3h and 3i, 92 and 50%, respectively (entries 8 and 9).
It should be noted that starting compounds 1/2, bearing both electron withdrawing and donating substituents R1, R2 in aryl rings Ar1, Ar2, respectively, led to the exclusive formation of indenes 3, formed by the cyclization at carbon C4 in CF3-allyl cations A (see Fig. 1). The formation of alternative indene structures by cyclization at carbon C2 in species A was not observed at all. This regioselectivity in reactions of CF3-cations A is contrary to the non-selective behaviour of other allyl cations without the CF3-substituent.20
Despite that indenes 3 were formed quantitatively without any further purification, under the attempt of their additional column chromatography isolation with silica gel, the isomerization into other indenes 4 was observed (Scheme 2). Thus, we developed the procedure for this quantitative isomerization by stirring a solution of compounds 3 in EtOAc in the presence of silica gel at room temperature for 4 h (see Scheme 2 for selected indenes 3/4). Compared with compounds 3, their isomers 4 should be thermodynamically more stable, because of the additional conjugation of the indene C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3 double bond with the aryl group Ar2. Some of the indenes 3 were isomerized very easily. Thus, under the isolation (without column chromatography with silica gel) of 3q, the additional formation of the corresponding isomeric indene 4q was observed (entry 17, Table 1). On the other hand, polymethylated indenes 3j2, 3l and 3m, bearing a methyl group in the position 7 of the indene system, were not isomerized at all, presumably, because of the steric hindrance from this methyl substituent. Most likely, the presence of the substituent in the indene position 7 is a crucial point for this isomerization. Any bulky group in this position may disturb a flat orientation of ring Ar2 relatively to the indene plane, which is thermodynamically favorable for conjugation of their π-systems. A shift of the double bond in the indene system with a formation of isomeric indenes has also been observed under the action of various basic or acidic reagents.13,21–23
C3 double bond with the aryl group Ar2. Some of the indenes 3 were isomerized very easily. Thus, under the isolation (without column chromatography with silica gel) of 3q, the additional formation of the corresponding isomeric indene 4q was observed (entry 17, Table 1). On the other hand, polymethylated indenes 3j2, 3l and 3m, bearing a methyl group in the position 7 of the indene system, were not isomerized at all, presumably, because of the steric hindrance from this methyl substituent. Most likely, the presence of the substituent in the indene position 7 is a crucial point for this isomerization. Any bulky group in this position may disturb a flat orientation of ring Ar2 relatively to the indene plane, which is thermodynamically favorable for conjugation of their π-systems. A shift of the double bond in the indene system with a formation of isomeric indenes has also been observed under the action of various basic or acidic reagents.13,21–23
 | ||
| Scheme 2 Isomerization of CF3-indenes 3 into 4 (see substituents R1, R2 in aryl rings Ar1, Ar2, respectively, in Table 1). | ||
Conclusion
We have found a novel, effective and simple method for the synthesis of two series of isomeric CF3-indenes based on acid (H2SO4 or CF3CO2H)-promoted cyclization of 2,4-diaryl-1,1,1-trifluorobut-3-en-2-oles or their TMS-ethers.Experimental part
Instruments
The NMR spectra of solutions of compounds in CDCl3 were recorded on a Bruker AVANCE III 400 (at 400, 376 and 100 MHz for 1H, 19F and 13C NMR spectra, respectively) spectrometer at 25 °C. The residual proton-solvent peak CDCl3 (δ 7.26 ppm) for 1H NMR spectra and the carbon signal of CDCl3 (δ 77.0 ppm) for 13C NMR spectra were used as references. 19F NMR spectra were indirectly referred to the signal of CFCl3 (δ 0.0 ppm). HRMS was carried out with a Bruker maXis HRMS-ESI-QTOF and a Varian 902-MS MALDI mass spectrometer. Chromato-mass-spectrometry data were obtained using a Shimadzu QP-2010 Ultra equipped with a SPB-1 SULFUR capillary column (30 m × 0.32 mm), the thickness of the stationary phase being 1.25 μm. The preparative reactions were monitored by thin-layer chromatography carried out on silica gel plates (Alugram SIL G/UV-254), using UV light for detection. Preparative TLC was performed on silica gel Chemapol L 5/40 with the petroleum ether–ethyl acetate mixture as an eluent.DFT calculations
All computations were carried out at the DFT/HF hybrid level of theory using the Becke's three-parameter hybrid exchange functional in combination with the gradient-corrected correlation functional of Lee, Yang, and Parr (B3LYP) by using GAUSSIAN 2009 program packages.24 The geometry optimization was performed using the 6-311+G(2d,2p) basis set (standard 6-311 basis set added with polarization (d, p) and diffuse functions). Optimizations were performed on all degrees of freedom, and solvent-phase optimized structures were verified as true minima with no imaginary frequencies. The Hessian matrix was calculated analytically for the optimized structures in order to prove the location of correct minima and to estimate the thermodynamic parameters. Gibbs free energies were calculated at 25 °C. Solvent-phase calculations used the Polarizable Continuum Model (PCM).Synthesis and characterization of compounds 1 and 2
Trifluoromethylation of chalcones with CF3SiMe3 leading to compounds 1 was carried out according to the literature procedures.25,26 Detrimethylsilylation of compounds 1 giving alcohols 2 was carried out with aqueous HCl25 or with SnCl2.27![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.71 d (1H,
CH, J 16.3 Hz), 6.71 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 7.29–7.43 m (8Harom.), 7.60–7.62 m (2Harom.). 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.40 s (CF3). HRMS: C19H21F3OSiAg found 457.0360 [M + Ag]+; calcd 457.0359.
CH, J 16.3 Hz), 7.29–7.43 m (8Harom.), 7.60–7.62 m (2Harom.). 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.40 s (CF3). HRMS: C19H21F3OSiAg found 457.0360 [M + Ag]+; calcd 457.0359.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.72 d (1H,
CH, J 16.3 Hz), 6.72 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 7.21 d (2Harom., J 8.1 Hz), 7.29–7.43 m (5Harom.), 7.49 d (2Harom., J 8.1 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 21.2 (Me), 80.0 q (C2, JC–F 28.8 Hz), 125.2 q (CF3, JC–F 286.8 Hz), 127.0, 127.2, 128.0, 128.7, 128.8, 128.9, 135.2, 135.2, 135.9, 138.5. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.57 s (CF3). HRMS: C20H23F3OSiAg found 471.0517 [M + Ag]+; calcd 471.0516.
CH, J 16.3 Hz), 7.21 d (2Harom., J 8.1 Hz), 7.29–7.43 m (5Harom.), 7.49 d (2Harom., J 8.1 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 21.2 (Me), 80.0 q (C2, JC–F 28.8 Hz), 125.2 q (CF3, JC–F 286.8 Hz), 127.0, 127.2, 128.0, 128.7, 128.8, 128.9, 135.2, 135.2, 135.9, 138.5. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.57 s (CF3). HRMS: C20H23F3OSiAg found 471.0517 [M + Ag]+; calcd 471.0516.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.4 Hz), 6.66 d (1H,
CH, J 16.4 Hz), 6.66 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.4 Hz), 7.17 d (2Harom., J 8.0 Hz), 7.31 d (2Harom., J 8.0 Hz), 7.36–7.43 m (3Harom.), 7.60–7.62 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 21.4 (Me), 80.1 q (C2, JC–F 28.8 Hz), 125.2 q (CF3, JC–F 286.6 Hz), 126.0, 126.9, 128.0, 128.1, 128.6, 129.7, 133.1, 135.4, 138.2, 138.8. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.57 s (CF3). HRMS: C20H23F3OSiAg found 471.0507 [M + Ag]+; calcd 471.0516.
CH, J 16.4 Hz), 7.17 d (2Harom., J 8.0 Hz), 7.31 d (2Harom., J 8.0 Hz), 7.36–7.43 m (3Harom.), 7.60–7.62 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 21.4 (Me), 80.1 q (C2, JC–F 28.8 Hz), 125.2 q (CF3, JC–F 286.6 Hz), 126.0, 126.9, 128.0, 128.1, 128.6, 129.7, 133.1, 135.4, 138.2, 138.8. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.57 s (CF3). HRMS: C20H23F3OSiAg found 471.0507 [M + Ag]+; calcd 471.0516.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.67 d (1H,
CH, J 16.3 Hz), 6.67 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 7.17 d (2Harom., J 8.0 Hz), 7.20 d (2Harom., J 8.1 Hz), 7.31 d (2Harom., J 8.0 Hz), 7.49 d (2Harom., J 8.1 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 21.2 (Me), 21.4 (Me), 80.0 q (C2, JC–F 28.7 Hz), 125.2 q (CF3, JC–F 286.6 Hz), 126.1, 126.9, 128.1, 128.8, 129.6, 133.2, 135.2, 135.3, 138.4, 138.7. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.75 c (CF3). HRMS: C21H25F3OSiAg found 485.0668 [M + Ag]+; calcd 485.0672.
CH, J 16.3 Hz), 7.17 d (2Harom., J 8.0 Hz), 7.20 d (2Harom., J 8.1 Hz), 7.31 d (2Harom., J 8.0 Hz), 7.49 d (2Harom., J 8.1 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 21.2 (Me), 21.4 (Me), 80.0 q (C2, JC–F 28.7 Hz), 125.2 q (CF3, JC–F 286.6 Hz), 126.1, 126.9, 128.1, 128.8, 129.6, 133.2, 135.2, 135.3, 138.4, 138.7. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.75 c (CF3). HRMS: C21H25F3OSiAg found 485.0668 [M + Ag]+; calcd 485.0672.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.64 d (1H,
CH, J 16.3 Hz), 6.64 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.91 d (2Harom., J 8.7 Hz), 7.37 d (2Harom., J 8.7 Hz), 7.40–7.42 m (3Harom.), 7.63–7.65 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 55.4 (OMe), 80.2 q (C2, J 28.8 Hz), 114.4, 124.7, 125.2 q (CF3, J 286.5 Hz), 128.0, 128.2, 128.3, 128.6, 128.6, 135.1, 138.3, 160.2. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.64 c (CF3). HRMS: C20H23F3O2SiAg found 487.0469 [M + Ag]+; calcd 487.0465.
CH, J 16.3 Hz), 6.91 d (2Harom., J 8.7 Hz), 7.37 d (2Harom., J 8.7 Hz), 7.40–7.42 m (3Harom.), 7.63–7.65 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 55.4 (OMe), 80.2 q (C2, J 28.8 Hz), 114.4, 124.7, 125.2 q (CF3, J 286.5 Hz), 128.0, 128.2, 128.3, 128.6, 128.6, 135.1, 138.3, 160.2. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.64 c (CF3). HRMS: C20H23F3O2SiAg found 487.0469 [M + Ag]+; calcd 487.0465.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.73 d (1H,
CH, J 16.3 Hz), 6.73 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.93 d (2Harom., J 8.6 Hz), 7.30–7.37 m (3Harom.), 7.42–7.44 m (2Harom.), 7.53 d (2Harom., J 8.6 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 55.4 (OMe), 79.8 q (C2, JC–F 28.9 Hz), 113.4, 125.2 q (CF3, JC–F 286.5 Hz), 127.0, 127.2, 128.7, 128.9, 129.5, 130.0, 135.2, 135.9, 159.9. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.73 c (CF3). HRMS: C20H23F3O2SiAg found 487.0477 [M + Ag]+; calcd 487.0465.
CH, J 16.3 Hz), 6.93 d (2Harom., J 8.6 Hz), 7.30–7.37 m (3Harom.), 7.42–7.44 m (2Harom.), 7.53 d (2Harom., J 8.6 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 55.4 (OMe), 79.8 q (C2, JC–F 28.9 Hz), 113.4, 125.2 q (CF3, JC–F 286.5 Hz), 127.0, 127.2, 128.7, 128.9, 129.5, 130.0, 135.2, 135.9, 159.9. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.73 c (CF3). HRMS: C20H23F3O2SiAg found 487.0477 [M + Ag]+; calcd 487.0465.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.71 d (1H,
CH, J 16.3 Hz), 6.71 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.88 d (1Harom., J 9.0 Hz), 7.14–7.15 m (2Harom.), 7.26–7.43 m (5Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 s (SiMe3), 56.0 (OMe), 56.0 (OMe), 79.9 q (C2, JC–F 28.7 Hz), 110.5, 111.6, 120.8, 125.2 q (CF3, JC–F 287.0 Hz), 127.0, 127.0, 128.8, 129.0, 130.4, 135.4, 135.9, 148.5, 149.3. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.64 c (CF3). HRMS: C21H25F3O2SiAg found 517.0559 [M + Ag]+; calcd 517.0571.
CH, J 16.3 Hz), 6.88 d (1Harom., J 9.0 Hz), 7.14–7.15 m (2Harom.), 7.26–7.43 m (5Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 s (SiMe3), 56.0 (OMe), 56.0 (OMe), 79.9 q (C2, JC–F 28.7 Hz), 110.5, 111.6, 120.8, 125.2 q (CF3, JC–F 287.0 Hz), 127.0, 127.0, 128.8, 129.0, 130.4, 135.4, 135.9, 148.5, 149.3. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.64 c (CF3). HRMS: C21H25F3O2SiAg found 517.0559 [M + Ag]+; calcd 517.0571.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.62 d (1H,
CH, J 16.3 Hz), 6.62 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.89 d (2Harom., J 8.7 Hz), 6.92 d (2Harom., J 8.8 Hz), 7.35 d (2Harom., J 8.7 Hz), 7.52 d (2Harom., J 8.8 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 55.4 (OMe), 55.5 (OMe), 79.9 q (C2, JC–F 29.0 Hz), 113.4, 114.4, 124.9, 125.3 q (CF3, JC–F 286.6 Hz), 128.3, 128.6, 129.5, 130.2, 134.9, 159.8, 160.2. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.02 c (CF3). HRMS: C21H25F3O2SiAg found 517.0550 [M + Ag]+; calcd 517.0571.
CH, J 16.3 Hz), 6.89 d (2Harom., J 8.7 Hz), 6.92 d (2Harom., J 8.8 Hz), 7.35 d (2Harom., J 8.7 Hz), 7.52 d (2Harom., J 8.8 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 55.4 (OMe), 55.5 (OMe), 79.9 q (C2, JC–F 29.0 Hz), 113.4, 114.4, 124.9, 125.3 q (CF3, JC–F 286.6 Hz), 128.3, 128.6, 129.5, 130.2, 134.9, 159.8, 160.2. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.02 c (CF3). HRMS: C21H25F3O2SiAg found 517.0550 [M + Ag]+; calcd 517.0571.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.61 d (1H,
CH, J 16.3 Hz), 6.61 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.85 d (1Harom., J 8.3 Hz), 6.93 d (1Harom., J 1.9 Hz), 6.96 dd (1Harom., J 8.3 Hz, J 1.9 Hz), 7.21 d (2Harom., J 8.1 Hz), 7.49 d (2Harom., J 8.1 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 21.2 (Me), 56.1 (OMe), 56.1 (OMe), 80.1 q (C2, J 28.8 Hz), 104.4, 109.4, 111.4, 120.3, 125.1, 125.2 q (CF3, JC–F 286.5 Hz), 128.1, 128.8, 128.9, 135.1, 135.2, 138.4, 149.4, 149.8. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.68 (CF3). HRMS: C22H27F3O3SiAg found 531.0727 [M + Ag]+; calcd 531.0731.
CH, J 16.3 Hz), 6.85 d (1Harom., J 8.3 Hz), 6.93 d (1Harom., J 1.9 Hz), 6.96 dd (1Harom., J 8.3 Hz, J 1.9 Hz), 7.21 d (2Harom., J 8.1 Hz), 7.49 d (2Harom., J 8.1 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 21.2 (Me), 56.1 (OMe), 56.1 (OMe), 80.1 q (C2, J 28.8 Hz), 104.4, 109.4, 111.4, 120.3, 125.1, 125.2 q (CF3, JC–F 286.5 Hz), 128.1, 128.8, 128.9, 135.1, 135.2, 138.4, 149.4, 149.8. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.68 (CF3). HRMS: C22H27F3O3SiAg found 531.0727 [M + Ag]+; calcd 531.0731.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.73 d (1H,
CH, J 16.3 Hz), 6.73 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 7.16 d (1Harom., J 7.8 Hz), 7.29–7.39 m (5Harom.), 7.42–7.44 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 19.6 (Me), 20.2 (Me), 80.0 q (C2, JC–F 28.8 Hz), 125.2 q (CF3, JC–F 286.7 Hz), 125.5, 127.0, 127.3, 128.7, 128.9, 129.2, 129.4, 135.0, 135.5, 136.0, 136.2, 137.1. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.41 c (CF3). HRMS: C21H25F3OSiAg found 485.0668 [M + Ag]+; calcd 485.0672.
CH, J 16.3 Hz), 7.16 d (1Harom., J 7.8 Hz), 7.29–7.39 m (5Harom.), 7.42–7.44 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 19.6 (Me), 20.2 (Me), 80.0 q (C2, JC–F 28.8 Hz), 125.2 q (CF3, JC–F 286.7 Hz), 125.5, 127.0, 127.3, 128.7, 128.9, 129.2, 129.4, 135.0, 135.5, 136.0, 136.2, 137.1. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.41 c (CF3). HRMS: C21H25F3OSiAg found 485.0668 [M + Ag]+; calcd 485.0672.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.4 Hz), 6.67 d (1H,
CH, J 16.4 Hz), 6.67 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.4 Hz), 7.01–7.03 m (2Harom.), 7.27–7.42 m (5Harom.), 7.50 d (1Harom., J 7.7 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 1.8 (SiMe3), 21.0 (Me), 22.6 (Me), 81.0 q (C2, JC–F 28.5 Hz), 125.6 q (CF3, JC–F 287.4 Hz), 126.2, 126.9, 128.3, 128.5, 128.9, 133.7, 133.8, 134.1, 136.2, 138.0, 138.3. 19F NMR (CDCl3, 376 MHz) δ, ppm: −74.50 c (CF3). HRMS: C21H25F3OSiAg found 485.0664 [M + Ag]+; calcd 485.0672.
CH, J 16.4 Hz), 7.01–7.03 m (2Harom.), 7.27–7.42 m (5Harom.), 7.50 d (1Harom., J 7.7 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 1.8 (SiMe3), 21.0 (Me), 22.6 (Me), 81.0 q (C2, JC–F 28.5 Hz), 125.6 q (CF3, JC–F 287.4 Hz), 126.2, 126.9, 128.3, 128.5, 128.9, 133.7, 133.8, 134.1, 136.2, 138.0, 138.3. 19F NMR (CDCl3, 376 MHz) δ, ppm: −74.50 c (CF3). HRMS: C21H25F3OSiAg found 485.0664 [M + Ag]+; calcd 485.0672.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.4 Hz), 6.68 d (1H,
CH, J 16.4 Hz), 6.68 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.4 Hz), 7.08 m (2Harom.), 7.28–7.37 m (3Harom.), 7.40–7.42 m (3Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 1.7 (SiMe3), 21.3 (Me), 22.2 (Me), 81.0 q (C2, JC–F 28.6 Hz), 125.5 q (CF3, JC–F 287.6 Hz), 126.8, 128.1, 128.4, 128.8, 129.0, 129.2, 132.8, 134.1, 134.7, 134.9, 136.1, 136.2. 19F NMR (CDCl3, 376 MHz) δ, ppm: −74.24 c (CF3). HRMS: C21H25F3OSiAg found 485.0662 [M + Ag]+; calcd 485.0672.
CH, J 16.4 Hz), 7.08 m (2Harom.), 7.28–7.37 m (3Harom.), 7.40–7.42 m (3Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 1.7 (SiMe3), 21.3 (Me), 22.2 (Me), 81.0 q (C2, JC–F 28.6 Hz), 125.5 q (CF3, JC–F 287.6 Hz), 126.8, 128.1, 128.4, 128.8, 129.0, 129.2, 132.8, 134.1, 134.7, 134.9, 136.1, 136.2. 19F NMR (CDCl3, 376 MHz) δ, ppm: −74.24 c (CF3). HRMS: C21H25F3OSiAg found 485.0662 [M + Ag]+; calcd 485.0672.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.4 Hz), 6.69 d (1H,
CH, J 16.4 Hz), 6.69 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.4 Hz), 6.97 s (1Harom.), 7.27–7.43 m (6Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 1.7 (SiMe3), 19.2 (Me), 19.5 (Me), 22.0 (Me), 80.8 q (C2, JC–F 28.5 Hz), 125.4 q (CF3, JC–F 287.6 Hz), 126.7, 128.2, 128.3, 128.7, 129.6, 133.2, 133.6, 133.9, 134.2, 135.1, 136.1, 136.7. 19F NMR (CDCl3, 376 MHz) δ, ppm: −74.54 c (CF3). HRMS: C22H27F3OSiAg found 499.0834 [M + Ag]+; calcd 499.0829.
CH, J 16.4 Hz), 6.97 s (1Harom.), 7.27–7.43 m (6Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 1.7 (SiMe3), 19.2 (Me), 19.5 (Me), 22.0 (Me), 80.8 q (C2, JC–F 28.5 Hz), 125.4 q (CF3, JC–F 287.6 Hz), 126.7, 128.2, 128.3, 128.7, 129.6, 133.2, 133.6, 133.9, 134.2, 135.1, 136.1, 136.7. 19F NMR (CDCl3, 376 MHz) δ, ppm: −74.54 c (CF3). HRMS: C22H27F3OSiAg found 499.0834 [M + Ag]+; calcd 499.0829.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.2 Hz), 6.59 d (1H,
CH, J 16.2 Hz), 6.59 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.2 Hz), 6.78 d (1Harom., J 8.0 Hz), 6.83 d (1Harom., J 8.0 Hz), 6.96 s (1Harom.), 7.36–7.42 m (3Harom.), 7.59–7.61 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 80.1 q (C2, JC–F 28.8 Hz), 101.4, 105.9, 108.6, 122.2, 125.2 q (CF3, JC–F 286.3 Hz), 125.2, 128.0, 128.1, 128.6, 130.3, 135.2, 138.2, 148.3, 148.5. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.59 c (CF3). HRMS: C20H21F3O3SiAg found 501.0252 [M + Ag]+; calcd 501.0258.
CH, J 16.2 Hz), 6.78 d (1Harom., J 8.0 Hz), 6.83 d (1Harom., J 8.0 Hz), 6.96 s (1Harom.), 7.36–7.42 m (3Harom.), 7.59–7.61 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.2 (SiMe3), 80.1 q (C2, JC–F 28.8 Hz), 101.4, 105.9, 108.6, 122.2, 125.2 q (CF3, JC–F 286.3 Hz), 125.2, 128.0, 128.1, 128.6, 130.3, 135.2, 138.2, 148.3, 148.5. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.59 c (CF3). HRMS: C20H21F3O3SiAg found 501.0252 [M + Ag]+; calcd 501.0258.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.67 d (1H,
CH, J 16.3 Hz), 6.67 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 7.34 s (4Harom.), 7.38–7.43 m (3Harom.), 7.59–7.61 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.1 (SiMe3), 80.1 q (C2, JC–F 29.0 Hz), 125.1 q (CF3, JC–F 286.8 Hz), 127.9, 128.0, 128.2, 128.2, 128.8, 129.2, 133.9, 134.4, 134.6, 138.0. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.19 c (CF3). HRMS: C21H25F335ClOSiAg found 490.9965 [M + Ag]+; calcd 490.9969.
CH, J 16.3 Hz), 7.34 s (4Harom.), 7.38–7.43 m (3Harom.), 7.59–7.61 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.1 (SiMe3), 80.1 q (C2, JC–F 29.0 Hz), 125.1 q (CF3, JC–F 286.8 Hz), 127.9, 128.0, 128.2, 128.2, 128.8, 129.2, 133.9, 134.4, 134.6, 138.0. 19F NMR (CDCl3, 376 MHz) δ, ppm: −77.19 c (CF3). HRMS: C21H25F335ClOSiAg found 490.9965 [M + Ag]+; calcd 490.9969.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.4 Hz), 6.61 d (1H,
CH, J 16.4 Hz), 6.61 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.4 Hz), 7.05–7.18 m (5Harom.), 7.59 dd (2Harom., J 8.6 Hz, J 5.5 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.1 (SiMe3), 19.6 (Me), 19.8 (Me), 79.8 q (C2, JC–F 29.0 Hz), 114.8 d (JC–F 21.5 Hz), 124.3, 125.1 q (CF3, JC–F 286.6 Hz), 125.3, 128.3, 130.1 d (J 8.2 Hz), 130.2, 133.2, 134.1 d (JC–F 3.1 Hz), 135.9, 137.2, 137.7, 162.9 d (JC–F 247.6 Hz). 19F NMR (CDCl3, 376 MHz) δ, ppm: −113.96 c (1Farom.), −77.95 c (CF3). HRMS: C21H24F4OSiAg found 503.0570 [M + Ag]+; calcd 503.0578.
CH, J 16.4 Hz), 7.05–7.18 m (5Harom.), 7.59 dd (2Harom., J 8.6 Hz, J 5.5 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.1 (SiMe3), 19.6 (Me), 19.8 (Me), 79.8 q (C2, JC–F 29.0 Hz), 114.8 d (JC–F 21.5 Hz), 124.3, 125.1 q (CF3, JC–F 286.6 Hz), 125.3, 128.3, 130.1 d (J 8.2 Hz), 130.2, 133.2, 134.1 d (JC–F 3.1 Hz), 135.9, 137.2, 137.7, 162.9 d (JC–F 247.6 Hz). 19F NMR (CDCl3, 376 MHz) δ, ppm: −113.96 c (1Farom.), −77.95 c (CF3). HRMS: C21H24F4OSiAg found 503.0570 [M + Ag]+; calcd 503.0578.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.57 d (1H,
CH, J 16.3 Hz), 6.57 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.3 Hz), 6.85 d (1Harom., J 8.3 Hz), 6.92 d (1Harom., J 1.9 Hz), 6.96 dd (1Harom., J 8.3 Hz, J 1.9 Hz), 7.05–7.11 m (2Harom.), 7.58 dd (2Harom., J 8.6 Hz, J 5.4 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.1 s (SiMe3), 56.1 (OMe), 56.1 (OMe), 79.8 q (C2, JC–F 29.0 Hz), 109.3, 111.4, 114.9 d (JC–F 21.5 Hz), 120.4, 124.6, 125.1 q (CF3, JC–F 286.3 Hz), 128.6, 130.1 d (JC–F 8.3 Hz), 134.0 d (JC–F 3.2 Hz), 135.6, 149.4, 150.0, 163.0 d (JC–F 247.7 Hz). 19F NMR (CDCl3, 376 MHz) δ, ppm: −113.88 c (1Farom.), −77.90 c (CF3). HRMS: C21H24F4O3SiAg found 535.0484 [M + Ag]+; calcd 535.0476.
CH, J 16.3 Hz), 6.85 d (1Harom., J 8.3 Hz), 6.92 d (1Harom., J 1.9 Hz), 6.96 dd (1Harom., J 8.3 Hz, J 1.9 Hz), 7.05–7.11 m (2Harom.), 7.58 dd (2Harom., J 8.6 Hz, J 5.4 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 2.1 s (SiMe3), 56.1 (OMe), 56.1 (OMe), 79.8 q (C2, JC–F 29.0 Hz), 109.3, 111.4, 114.9 d (JC–F 21.5 Hz), 120.4, 124.6, 125.1 q (CF3, JC–F 286.3 Hz), 128.6, 130.1 d (JC–F 8.3 Hz), 134.0 d (JC–F 3.2 Hz), 135.6, 149.4, 150.0, 163.0 d (JC–F 247.7 Hz). 19F NMR (CDCl3, 376 MHz) δ, ppm: −113.88 c (1Farom.), −77.90 c (CF3). HRMS: C21H24F4O3SiAg found 535.0484 [M + Ag]+; calcd 535.0476.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 6.89 d (1H,
CH, J 16.1 Hz), 6.89 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 7.28–7.45 m (8Harom.), 7.65–7.67 m (2Harom.). 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.50 c (CF3). HRMS: C16H13F3OAg found 384.9957 [M + Ag]+; calcd 384.9964.
CH, J 16.1 Hz), 7.28–7.45 m (8Harom.), 7.65–7.67 m (2Harom.). 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.50 c (CF3). HRMS: C16H13F3OAg found 384.9957 [M + Ag]+; calcd 384.9964.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 6.89 d (1H,
CH, J 16.1 Hz), 6.89 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 7.23 d (2Harom., J 8.1 Hz), 7.28–7.38 m (3Harom.), 7.43–7.45 m (2Harom.), 7.54 d (2Harom., J 8.1 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.21 (Me), 77.4 q (C2, JC–F 29.0 Hz), 125.2 q (CF3, JC–F 286.0 Hz), 126.7, 126.9, 126.9, 127.1, 128.7, 128.9, 129.2, 133.5, 134.6, 135.7, 138.9. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.57 c (CF3). HRMS: C17H15F3OAg found 399.0124 [M + Ag]+; calcd 399.0120.
CH, J 16.1 Hz), 7.23 d (2Harom., J 8.1 Hz), 7.28–7.38 m (3Harom.), 7.43–7.45 m (2Harom.), 7.54 d (2Harom., J 8.1 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.21 (Me), 77.4 q (C2, JC–F 29.0 Hz), 125.2 q (CF3, JC–F 286.0 Hz), 126.7, 126.9, 126.9, 127.1, 128.7, 128.9, 129.2, 133.5, 134.6, 135.7, 138.9. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.57 c (CF3). HRMS: C17H15F3OAg found 399.0124 [M + Ag]+; calcd 399.0120.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 6.85 d (1H,
CH, J 16.1 Hz), 6.85 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 7.16 d (2Harom., J 8.0 Hz), 7.33 d (2Harom., J 8.0 Hz), 7.37–7.44 m (3Harom.), 7.65–7.67 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.4 (Me), 77.5 q (C2, JC–F 29.0 Hz), 125.2 q (CF3, JC–F 286.0 Hz), 125.6, 127.0, 127.0, 128.5, 128.9, 129.6, 132.9, 133.6, 137.6, 138.8. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.55 c (CF3). HRMS: C17H15F3OAg found 399.0110 [M + Ag]+; calcd 399.0120.
CH, J 16.1 Hz), 7.16 d (2Harom., J 8.0 Hz), 7.33 d (2Harom., J 8.0 Hz), 7.37–7.44 m (3Harom.), 7.65–7.67 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.4 (Me), 77.5 q (C2, JC–F 29.0 Hz), 125.2 q (CF3, JC–F 286.0 Hz), 125.6, 127.0, 127.0, 128.5, 128.9, 129.6, 132.9, 133.6, 137.6, 138.8. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.55 c (CF3). HRMS: C17H15F3OAg found 399.0110 [M + Ag]+; calcd 399.0120.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 6.84 d (1H,
CH, J 16.1 Hz), 6.84 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 7.16 d (2Harom., J 8.0 Hz), 7.22 d (2Harom., J 8.1 Hz), 7.32 d (2Harom., J 8.0 Hz), 7.53 d (2Harom., J 8.1 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.2 (Me), 21.4 (Me), 77.4 q (C2, JC–F 28.6 Hz), 125.3 q (CF3, J 286.2 Hz), 125.7, 126.9, 127.0, 129.2, 129.6, 132.9, 133.5, 134.7, 138.7, 138.8. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.65 c (CF3). HRMS: C18H17F3OAg found 413.0267 [M + Ag]+; calcd 413.0277.
CH, J 16.1 Hz), 7.16 d (2Harom., J 8.0 Hz), 7.22 d (2Harom., J 8.1 Hz), 7.32 d (2Harom., J 8.0 Hz), 7.53 d (2Harom., J 8.1 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.2 (Me), 21.4 (Me), 77.4 q (C2, JC–F 28.6 Hz), 125.3 q (CF3, J 286.2 Hz), 125.7, 126.9, 127.0, 129.2, 129.6, 132.9, 133.5, 134.7, 138.7, 138.8. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.65 c (CF3). HRMS: C18H17F3OAg found 413.0267 [M + Ag]+; calcd 413.0277.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 6.81 d (1H,
CH, J 16.1 Hz), 6.81 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 6.88 d (2Harom., J 8.8 Hz), 7.35–7.44 m (5Harom.), 7.64–7.66 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 55.5 (OMe), 77.5 q (C2, JC–F 28.6 Hz), 114.3, 124.4, 125.3 q (CF3, J 285.9 Hz), 127.0, 128.4, 128.4, 128.5, 128.9, 133.3, 137.7, 160.2. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.57 c (CF3). HRMS: C17H15F3O2Ag found 415.0089 [M + Ag]+; calcd 415.0070.
CH, J 16.1 Hz), 6.88 d (2Harom., J 8.8 Hz), 7.35–7.44 m (5Harom.), 7.64–7.66 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 55.5 (OMe), 77.5 q (C2, JC–F 28.6 Hz), 114.3, 124.4, 125.3 q (CF3, J 285.9 Hz), 127.0, 128.4, 128.4, 128.5, 128.9, 133.3, 137.7, 160.2. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.57 c (CF3). HRMS: C17H15F3O2Ag found 415.0089 [M + Ag]+; calcd 415.0070.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 6.80 d (1H,
CH, J 16.1 Hz), 6.80 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 6.87 d (2Harom., J 8.8 Hz), 7.35–7.43 m (5Harom.), 7.64–7.65 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 55.5 (OMe), 77.5 q (C2, JC–F 28.9 Hz), 114.3, 124.4, 125.3 q (CF3, JC–F 286.1 Hz), 127.0, 128.3, 128.4, 128.4, 128.8, 133.3, 137.8, 160.1. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.59 c (CF3). HRMS: C17H15F3O2Ag found 415.0073 [M + Ag]+; calcd 415.0070.
CH, J 16.1 Hz), 6.87 d (2Harom., J 8.8 Hz), 7.35–7.43 m (5Harom.), 7.64–7.65 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 55.5 (OMe), 77.5 q (C2, JC–F 28.9 Hz), 114.3, 124.4, 125.3 q (CF3, JC–F 286.1 Hz), 127.0, 128.3, 128.4, 128.4, 128.8, 133.3, 137.8, 160.1. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.59 c (CF3). HRMS: C17H15F3O2Ag found 415.0073 [M + Ag]+; calcd 415.0070.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 6.88 d (1H,
CH, J 16.1 Hz), 6.88 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 6.88 d (1Harom., J 8.5 Hz), 7.16 d (1Harom., J 8.5 Hz), 7.19 s (1Harom.), 7.28–7.37 m (3Harom.), 7.43 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 56.0 (OMe), 56.1 (OMe), 77.5 q (C2, J 28.6 Hz), 110.3, 110.8, 119.8, 125.3 q (CF3, JC–F 286.1 Hz), 126.7, 127.0, 128.8, 128.9, 129.9, 133.7, 135.7, 148.9, 149.5. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.54 c (CF3). HRMS: C18H17F3O3Ag found 445.0178 [M + Ag]+; calcd 445.0175.
CH, J 16.1 Hz), 6.88 d (1Harom., J 8.5 Hz), 7.16 d (1Harom., J 8.5 Hz), 7.19 s (1Harom.), 7.28–7.37 m (3Harom.), 7.43 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 56.0 (OMe), 56.1 (OMe), 77.5 q (C2, J 28.6 Hz), 110.3, 110.8, 119.8, 125.3 q (CF3, JC–F 286.1 Hz), 126.7, 127.0, 128.8, 128.9, 129.9, 133.7, 135.7, 148.9, 149.5. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.54 c (CF3). HRMS: C18H17F3O3Ag found 445.0178 [M + Ag]+; calcd 445.0175.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 6.79 d (1H,
CH, J 16.1 Hz), 6.79 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 6.87 d (2Harom., J 8.7 Hz), 6.92 d (2Harom., J 8.8 Hz), 7.36 d (2Harom., J 8.7 Hz), 7.56 d (2Harom., J 8.8 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 55.4 (OMe), 55.5 (OMe), 77.2 q (C2, JC–F 28.6 Hz), 113.8, 114.3, 124.5, 125.3 q (CF3, JC–F 286.0 Hz), 128.3, 128.5, 129.8, 133.1, 159.9, 160.1. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.77 c (CF3). HRMS: C18H17F3O3Ag found 445.0173 [M + Ag]+; calcd 445.0175.
CH, J 16.1 Hz), 6.87 d (2Harom., J 8.7 Hz), 6.92 d (2Harom., J 8.8 Hz), 7.36 d (2Harom., J 8.7 Hz), 7.56 d (2Harom., J 8.8 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 55.4 (OMe), 55.5 (OMe), 77.2 q (C2, JC–F 28.6 Hz), 113.8, 114.3, 124.5, 125.3 q (CF3, JC–F 286.0 Hz), 128.3, 128.5, 129.8, 133.1, 159.9, 160.1. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.77 c (CF3). HRMS: C18H17F3O3Ag found 445.0173 [M + Ag]+; calcd 445.0175.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.0 Hz), 6.79 d (1H,
CH, J 16.0 Hz), 6.79 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.0 Hz), 6.83 d (1Harom., J 8.1 Hz), 6.95–6.97 m (2Harom.), 7.22 d (2Harom., J 8.1 Hz), 7.53 d (2Harom., J 8.1 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.2 (Me), 56.1 (OMe), 56.1 (OMe), 77.4 q (C2, J 28.8 Hz), 109.4, 111.3, 120.5, 124.7, 125.3 q (CF3, JC–F 285.8 Hz), 126.9, 128.8, 129.2, 133.5, 134.8, 138.8, 149.3, 149.8. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.49 c (CF3). HRMS: C19H19F3O3Ag found 459.0328 [M + Ag]+; calcd 459.0332.
CH, J 16.0 Hz), 6.83 d (1Harom., J 8.1 Hz), 6.95–6.97 m (2Harom.), 7.22 d (2Harom., J 8.1 Hz), 7.53 d (2Harom., J 8.1 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.2 (Me), 56.1 (OMe), 56.1 (OMe), 77.4 q (C2, J 28.8 Hz), 109.4, 111.3, 120.5, 124.7, 125.3 q (CF3, JC–F 285.8 Hz), 126.9, 128.8, 129.2, 133.5, 134.8, 138.8, 149.3, 149.8. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.49 c (CF3). HRMS: C19H19F3O3Ag found 459.0328 [M + Ag]+; calcd 459.0332.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 6.89 d (1H,
CH, J 16.1 Hz), 6.89 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.1 Hz), 7.17 d (1Harom., J 8.0 Hz), 7.27–7.36 m (4Harom.), 7.40 s (1Harom.), 7.42–7.44 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 19.5 (Me), 20.1 (Me), 77.3 q (C2, JC–F 28.8 Hz), 124.3, 125.3 q (CF3, JC–F 286.0 Hz), 126.9, 127.0, 127.9, 128.6, 128.8, 129.7, 133.2, 135.1, 135.9, 136.8, 137.4. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.50 c (CF3). HRMS: C18H17F3OAg found 413.0269 [M + Ag]+; calcd 413.0277.
CH, J 16.1 Hz), 7.17 d (1Harom., J 8.0 Hz), 7.27–7.36 m (4Harom.), 7.40 s (1Harom.), 7.42–7.44 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 19.5 (Me), 20.1 (Me), 77.3 q (C2, JC–F 28.8 Hz), 124.3, 125.3 q (CF3, JC–F 286.0 Hz), 126.9, 127.0, 127.9, 128.6, 128.8, 129.7, 133.2, 135.1, 135.9, 136.8, 137.4. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.50 c (CF3). HRMS: C18H17F3OAg found 413.0269 [M + Ag]+; calcd 413.0277.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.2 Hz), 6.75 d (1H,
CH, J 16.2 Hz), 6.75 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.2 Hz), 6.99 s (1Harom.), 7.27–7.37 m (3Harom.), 7.42–7.43 m (3Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 19.3 (Me), 19.6 (Me), 22.0 q (Me, J 1.2 Hz), 78.9 q (C2, JC–F 28.6 Hz), 125.7 q (CF3, JC–F 286.7 Hz), 126.9, 127.5, 128.6, 128.9, 129.2, 132.8, 133.7, 134.0, 134.5, 135.1, 135.9, 137.4. 19F NMR (CDCl3, 376 MHz) δ, ppm: −76.72 c (CF3). HRMS: C19H19F3OAg found 427.0422 [M + Ag]+; calcd 427.0433.
CH, J 16.2 Hz), 6.99 s (1Harom.), 7.27–7.37 m (3Harom.), 7.42–7.43 m (3Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 19.3 (Me), 19.6 (Me), 22.0 q (Me, J 1.2 Hz), 78.9 q (C2, JC–F 28.6 Hz), 125.7 q (CF3, JC–F 286.7 Hz), 126.9, 127.5, 128.6, 128.9, 129.2, 132.8, 133.7, 134.0, 134.5, 135.1, 135.9, 137.4. 19F NMR (CDCl3, 376 MHz) δ, ppm: −76.72 c (CF3). HRMS: C19H19F3OAg found 427.0422 [M + Ag]+; calcd 427.0433.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.0 Hz), 6.77 d (1Harom., J 8.1 Hz), 6.77 d (1H,
CH, J 16.0 Hz), 6.77 d (1Harom., J 8.1 Hz), 6.77 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.0 Hz), 6.85 dd (1Harom., J 8.1 Hz, J 1.5 Hz), 6.97 d (1Harom., J 1.5 Hz), 7.36–7.43 m (3Harom.), 7.63–7.64 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 77.4 q (C2, J 29.2 Hz), 101.4, 106.1, 108.5, 122.2, 124.8, 125.2 q (CF3, JC–F 285.8 Hz), 127.0, 128.5, 128.9, 130.1, 133.4, 137.6, 148.2, 148.4. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.57 c (CF3). HRMS: C17H13F3O3Ag found 428.9867 [M + Ag]+; calcd 428.9863.
CH, J 16.0 Hz), 6.85 dd (1Harom., J 8.1 Hz, J 1.5 Hz), 6.97 d (1Harom., J 1.5 Hz), 7.36–7.43 m (3Harom.), 7.63–7.64 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 77.4 q (C2, J 29.2 Hz), 101.4, 106.1, 108.5, 122.2, 124.8, 125.2 q (CF3, JC–F 285.8 Hz), 127.0, 128.5, 128.9, 130.1, 133.4, 137.6, 148.2, 148.4. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.57 c (CF3). HRMS: C17H13F3O3Ag found 428.9867 [M + Ag]+; calcd 428.9863.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.0 Hz), 6.85 d (1H,
CH, J 16.0 Hz), 6.85 d (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 16.0 Hz), 7.30–7.45 m (7Harom.), 7.63–7.65 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 77.5 q (C2, JC–F 29.1 Hz), 125.1 q (CF3, JC–F 286.0 Hz), 126.8, 127.2, 128.3, 128.6, 129.0, 132.5, 134.2, 134.5, 137.4. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.40 c (CF3). HRMS: C16H12F335ClOAg found 418.9557 [M + Ag]+; calcd 418.9574.
CH, J 16.0 Hz), 7.30–7.45 m (7Harom.), 7.63–7.65 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 77.5 q (C2, JC–F 29.1 Hz), 125.1 q (CF3, JC–F 286.0 Hz), 126.8, 127.2, 128.3, 128.6, 129.0, 132.5, 134.2, 134.5, 137.4. 19F NMR (CDCl3, 376 MHz) δ, ppm: −78.40 c (CF3). HRMS: C16H12F335ClOAg found 418.9557 [M + Ag]+; calcd 418.9574.
Synthesis and characterization of indenes 3
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.12 d (2Harom., J 7.6 Hz), 7.28–7.33 m (5Harom.), 7.38 t (1Harom., J 7.4 Hz), 7.57 d (1Harom., J 7.6 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 55.6 (C1), 121.2, 122.5 q (CF3, J 270.0 Hz), 123.9, 126.6, 127.0, 127.6, 128.03, 129.1, 134.6 q (C3, J 34.3 Hz), 137.1, 138.2, 141.0 q (C2, J 5.0 Hz), 148.0. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.05 s (CF3). HRMS (MALDI): C16H12F3 found 261.0886 [M + H]+, calcd 261.0891.
CH), 7.12 d (2Harom., J 7.6 Hz), 7.28–7.33 m (5Harom.), 7.38 t (1Harom., J 7.4 Hz), 7.57 d (1Harom., J 7.6 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 55.6 (C1), 121.2, 122.5 q (CF3, J 270.0 Hz), 123.9, 126.6, 127.0, 127.6, 128.03, 129.1, 134.6 q (C3, J 34.3 Hz), 137.1, 138.2, 141.0 q (C2, J 5.0 Hz), 148.0. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.05 s (CF3). HRMS (MALDI): C16H12F3 found 261.0886 [M + H]+, calcd 261.0891.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.12 d (2Harom., J 8.0 Hz), 7.13 c (1Harom.), 7.19 d (1Harom., J 7.8 Hz), 7.28–7.34 m (3Harom.), 7.44 d (1Harom., J 7.8 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.6 (Me), 55.4 (C1), 120.6, 122.6 q (CF3, J 270.0 Hz), 125.3, 127.5, 128.1, 128.3, 129.1, 134.5 q (C3, J 34.2 Hz), 135.5 d (C3a, J 1.1 Hz), 137.0, 137.4 d (C7a, J 0.8 Hz), 140.0 q (C2, J 5.1 Hz), 148.4. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.07 s (CF3). HRMS (MALDI): C17H14F3 found 275.1042 [M + H]+, calcd 275.1048.
CH), 7.12 d (2Harom., J 8.0 Hz), 7.13 c (1Harom.), 7.19 d (1Harom., J 7.8 Hz), 7.28–7.34 m (3Harom.), 7.44 d (1Harom., J 7.8 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.6 (Me), 55.4 (C1), 120.6, 122.6 q (CF3, J 270.0 Hz), 125.3, 127.5, 128.1, 128.3, 129.1, 134.5 q (C3, J 34.2 Hz), 135.5 d (C3a, J 1.1 Hz), 137.0, 137.4 d (C7a, J 0.8 Hz), 140.0 q (C2, J 5.1 Hz), 148.4. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.07 s (CF3). HRMS (MALDI): C17H14F3 found 275.1042 [M + H]+, calcd 275.1048.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.12 d (2Harom., J 8.1 Hz), 7.26–7.32 m (2Harom.), 7.37 t.d (1Harom., J 7.6 Hz, J 1.5 Hz), 7.56 d (1Harom., J 7.6 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.2 (Me), 55.3 (C1), 120.9, 122.6 q (CF3, J 270.0 Hz), 124.5, 126.9, 127.4, 127.9, 129.8, 133.9, 134.4 q (C3, J 34.2 Hz), 137.3, 138.2, 141.3 q (C2, J 5.0 Hz), 148.2. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.02 s (CF3). HRMS (MALDI): C17H14F3 found 275.1042 [M + H]+, calcd 275.1038.
CH), 7.12 d (2Harom., J 8.1 Hz), 7.26–7.32 m (2Harom.), 7.37 t.d (1Harom., J 7.6 Hz, J 1.5 Hz), 7.56 d (1Harom., J 7.6 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.2 (Me), 55.3 (C1), 120.9, 122.6 q (CF3, J 270.0 Hz), 124.5, 126.9, 127.4, 127.9, 129.8, 133.9, 134.4 q (C3, J 34.2 Hz), 137.3, 138.2, 141.3 q (C2, J 5.0 Hz), 148.2. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.02 s (CF3). HRMS (MALDI): C17H14F3 found 275.1042 [M + H]+, calcd 275.1038.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.00 d (2Harom., J 7.8 Hz), 7.12 s (1Harom.), 7.13 d (2Harom., J 7.8 Hz), 7.18 d (1Harom., J 7.9 Hz), 7.43 d (1Harom., J 7.9 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.2 (Me), 21.6 (Me), 55.1 (C1), 120.5, 122.6 q (CF3, J 270.0 Hz), 125.3, 127.9, 128.2, 129.8, 134.3, 134.3 q (C3, J 34.2 Hz), 135.5, 137.0, 137.2, 140.2 q (C2, J 5.1 Hz). 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.05 s (CF3). HRMS (MALDI): C18H16F3 found 289.1199 [M + H]+, calcd 289.1210.
CH), 7.00 d (2Harom., J 7.8 Hz), 7.12 s (1Harom.), 7.13 d (2Harom., J 7.8 Hz), 7.18 d (1Harom., J 7.9 Hz), 7.43 d (1Harom., J 7.9 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.2 (Me), 21.6 (Me), 55.1 (C1), 120.5, 122.6 q (CF3, J 270.0 Hz), 125.3, 127.9, 128.2, 129.8, 134.3, 134.3 q (C3, J 34.2 Hz), 135.5, 137.0, 137.2, 140.2 q (C2, J 5.1 Hz). 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.05 s (CF3). HRMS (MALDI): C18H16F3 found 289.1199 [M + H]+, calcd 289.1210.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.01 d (2Harom., J 8.6 Hz), 7.25–7.30 m (2Harom.), 7.37 m (1Harom.), 7.54 d (1Harom., J 7.5 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 54.9 (C1), 55.4 (OMe), 114.5, 120.9, 122.6 q (CF3, J 270.0 Hz), 124.5, 126.9, 127.4, 128.8, 129.0, 134.3 q (C3, J 34.2 Hz), 138.1, 141.4 q (C2, J 5.0 Hz), 148.3, 159.1. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.03 s (CF3). HRMS (MALDI): C17H14F3O found 291.0991 [M + H]+, calcd 291.0998.
CH), 7.01 d (2Harom., J 8.6 Hz), 7.25–7.30 m (2Harom.), 7.37 m (1Harom.), 7.54 d (1Harom., J 7.5 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 54.9 (C1), 55.4 (OMe), 114.5, 120.9, 122.6 q (CF3, J 270.0 Hz), 124.5, 126.9, 127.4, 128.8, 129.0, 134.3 q (C3, J 34.2 Hz), 138.1, 141.4 q (C2, J 5.0 Hz), 148.3, 159.1. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.03 s (CF3). HRMS (MALDI): C17H14F3O found 291.0991 [M + H]+, calcd 291.0998.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.02 d (2Harom., J 8.7 Hz), 7.25–7.31 m (2Harom.), 7.36 m (1Harom.), 7.55 d (1Harom., J 7.6 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 54.9 (C1), 55.4 (OMe), 114.5, 120.9, 122.6 q (CF3, J 270.0 Hz), 124.5, 126.9, 127.4, 128.8, 129.0, 134.3 q (C3, J 34.2 Hz), 138.1, 141.4 q (C2, J 5.0 Hz), 148.3, 159.1. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.02 s (CF3). HRMS (MALDI): C17H14F3O found 291.0991 [M + H]+, calcd 291.1002.
CH), 7.02 d (2Harom., J 8.7 Hz), 7.25–7.31 m (2Harom.), 7.36 m (1Harom.), 7.55 d (1Harom., J 7.6 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 54.9 (C1), 55.4 (OMe), 114.5, 120.9, 122.6 q (CF3, J 270.0 Hz), 124.5, 126.9, 127.4, 128.8, 129.0, 134.3 q (C3, J 34.2 Hz), 138.1, 141.4 q (C2, J 5.0 Hz), 148.3, 159.1. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.02 s (CF3). HRMS (MALDI): C17H14F3O found 291.0991 [M + H]+, calcd 291.1002.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.05 c (1Harom.), 7.08–7.10 m (2Harom.), 7.27–7.33 m (3Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 55.6 (C1), 56.3 (OMe), 56.4 (OMe), 104.0, 108.1, 122.6 q (CF3, J 269.9 Hz), 127.5, 128.0, 129.1, 130.8, 134.0 q (C3, J 34.1 Hz), 137.4, 139.8 q (C2, J 5.2 Hz), 140.9, 149.0. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −63.95 s (CF3). HRMS (MALDI): C18H16F3O2 found 321.1097 [M + H]+, calcd 321.1095.
CH), 7.05 c (1Harom.), 7.08–7.10 m (2Harom.), 7.27–7.33 m (3Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 55.6 (C1), 56.3 (OMe), 56.4 (OMe), 104.0, 108.1, 122.6 q (CF3, J 269.9 Hz), 127.5, 128.0, 129.1, 130.8, 134.0 q (C3, J 34.1 Hz), 137.4, 139.8 q (C2, J 5.2 Hz), 140.9, 149.0. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −63.95 s (CF3). HRMS (MALDI): C18H16F3O2 found 321.1097 [M + H]+, calcd 321.1095.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH + 1Harom.), 6.89 dd (1Harom., J 8.3 Hz, J 2.3 Hz), 7.01 d (2Harom., J 8.6 Hz), 7.42 d (Harom., J 8.4 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 54.8 (C1), 55.4 (OMe), 55.7 (OMe), 110.9, 113.0, 114.5, 121.4, 122.6 q (CF3, J 269.9 Hz), 129.0, 129.2, 131.0, 133.8 q (C3, J 34.1 Hz), 139.2 q (C2, J 5.1 Hz), 150.4, 159.1, 159.5. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.11 s (CF3). HRMS (MALDI): C18H16F3O2 found 321.1097 [M + H]+, calcd 321.1115.
CH + 1Harom.), 6.89 dd (1Harom., J 8.3 Hz, J 2.3 Hz), 7.01 d (2Harom., J 8.6 Hz), 7.42 d (Harom., J 8.4 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 54.8 (C1), 55.4 (OMe), 55.7 (OMe), 110.9, 113.0, 114.5, 121.4, 122.6 q (CF3, J 269.9 Hz), 129.0, 129.2, 131.0, 133.8 q (C3, J 34.1 Hz), 139.2 q (C2, J 5.1 Hz), 150.4, 159.1, 159.5. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.11 s (CF3). HRMS (MALDI): C18H16F3O2 found 321.1097 [M + H]+, calcd 321.1115.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.2–7.5 m (6Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.4 (Me), 42.2 (C1), 56.1 (2OMe), 111.3, 120.4, 123.5 q (CF3, J 273.3Hz), 125.3, 127.6, 129.8, 130.2, 139.2, 139.4 q (C3, J 34 Hz), 140.1 q (C2, J 5.0Hz), 148.6. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.04 s (CF3). HRMS (MALDI): C19H18F3O2 found 335.1253 [M + H]+, calcd 335.1267.
CH), 7.2–7.5 m (6Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.4 (Me), 42.2 (C1), 56.1 (2OMe), 111.3, 120.4, 123.5 q (CF3, J 273.3Hz), 125.3, 127.6, 129.8, 130.2, 139.2, 139.4 q (C3, J 34 Hz), 140.1 q (C2, J 5.0Hz), 148.6. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.04 s (CF3). HRMS (MALDI): C19H18F3O2 found 335.1253 [M + H]+, calcd 335.1267.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3j2 1.25
3j2 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Compound 3j1: 1H NMR (CDCl3, 400 MHz) (from the spectrum of a mixture of isomers) δ, ppm: 2.26 s (3H, Me), 2.34 s (3H, Me), 4.67 m (1H, C1H), 6.93 m (1H,
1). Compound 3j1: 1H NMR (CDCl3, 400 MHz) (from the spectrum of a mixture of isomers) δ, ppm: 2.26 s (3H, Me), 2.34 s (3H, Me), 4.67 m (1H, C1H), 6.93 m (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.08 c (1Harom.), 7.10–7.12 m (2Harom., J 1.5 Hz), 7.22 d (1Harom., J 7.7 Hz), 7.24–7.31 m (3Harom.). 13C NMR (CDCl3, 100 MHz) (from the spectrum of a mixture of isomers, some signals) δ, ppm: 20.1 (Me), 20.2 (Me), 55.2 (C1), 122.64 q (CF3, J 270.0 Hz), 134.5 q (C3, J 34.1 Hz), 140.1 q (C2, J 5.1 Hz). 19F {1H} NMR (CDCl3, 376 MHz) (from the spectrum of a mixture of isomers) δ, ppm: −64.00 s (CF3) Mass spectrum (GC-MS), m/z (IotH., %): 288 [M]+ (100). Compound 3j2: 1H NMR (CDCl3, 400 MHz) (from the spectrum of a mixture of isomers) δ, ppm: 1.99 c (3H, Me), 2.30 c (3H, Me), 4.72 m (1H, C1H), 6.86 m (1H,
CH), 7.08 c (1Harom.), 7.10–7.12 m (2Harom., J 1.5 Hz), 7.22 d (1Harom., J 7.7 Hz), 7.24–7.31 m (3Harom.). 13C NMR (CDCl3, 100 MHz) (from the spectrum of a mixture of isomers, some signals) δ, ppm: 20.1 (Me), 20.2 (Me), 55.2 (C1), 122.64 q (CF3, J 270.0 Hz), 134.5 q (C3, J 34.1 Hz), 140.1 q (C2, J 5.1 Hz). 19F {1H} NMR (CDCl3, 376 MHz) (from the spectrum of a mixture of isomers) δ, ppm: −64.00 s (CF3) Mass spectrum (GC-MS), m/z (IotH., %): 288 [M]+ (100). Compound 3j2: 1H NMR (CDCl3, 400 MHz) (from the spectrum of a mixture of isomers) δ, ppm: 1.99 c (3H, Me), 2.30 c (3H, Me), 4.72 m (1H, C1H), 6.86 m (1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.03–7.05 m (2Harom.), 7.24–7.31 m (4Harom.), 7.34 c (1Harom.). 13C NMR (CDCl3, 100 MHz) (from the spectrum of a mixture of isomers) δ, ppm: 15.8 (Me), 19.8 (Me), 55.4 (C1), 122.62 q (CF3, J 270.0 Hz), 133.4 q (C3, J 34.1 Hz), 140.9 q (C2, J 5.1 Hz). 19F {1H} NMR (CDCl3, 376 MHz) (from the spectrum of a mixture of isomers) δ, ppm: −64.10 s (CF3). For a mixture of isomers: 7.24–7.31 m (3Harom.A + 4Harom.B). Mass spectrum (GC-MS), m/z (IotH., %): 288 [M]+ (80). 13C NMR (CDCl3, 100 MHz) δ, ppm: 118.1, 121.9, 125.7, 127.3, 127.5, 128.0, 129.1, 129.5, 133.4, 135.6, 135.8, 135.9, 136.1, 136.7, 136.8, 137.7, 145.9, 146.1. HRMS (MALDI): C18H16F3 found 289.1199 [M + H]+, calcd 289.1204 (for a mixture of isomers).
CH), 7.03–7.05 m (2Harom.), 7.24–7.31 m (4Harom.), 7.34 c (1Harom.). 13C NMR (CDCl3, 100 MHz) (from the spectrum of a mixture of isomers) δ, ppm: 15.8 (Me), 19.8 (Me), 55.4 (C1), 122.62 q (CF3, J 270.0 Hz), 133.4 q (C3, J 34.1 Hz), 140.9 q (C2, J 5.1 Hz). 19F {1H} NMR (CDCl3, 376 MHz) (from the spectrum of a mixture of isomers) δ, ppm: −64.10 s (CF3). For a mixture of isomers: 7.24–7.31 m (3Harom.A + 4Harom.B). Mass spectrum (GC-MS), m/z (IotH., %): 288 [M]+ (80). 13C NMR (CDCl3, 100 MHz) δ, ppm: 118.1, 121.9, 125.7, 127.3, 127.5, 128.0, 129.1, 129.5, 133.4, 135.6, 135.8, 135.9, 136.1, 136.7, 136.8, 137.7, 145.9, 146.1. HRMS (MALDI): C18H16F3 found 289.1199 [M + H]+, calcd 289.1204 (for a mixture of isomers).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.15 d (2Harom., J 6.6 Hz), 7.30–7.37 m (3Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 20.0 q (Me, J 4.7 Hz), 21.2 (Me), 54.6 (C1), 122.9 q (CF3, J 269.4 Hz), 123.1, 127.5, 128.1, 129.1, 131.3, 131.4, 133.8, 134.5 q (C3, J 33.9 Hz), 137.0, 137.8, 141.8 q (C2, J 6.3 Hz), 149.8. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −60.61 s (CF3). HRMS (MALDI): C18H16F3 found 289.1199 [M + H]+, calcd 289.1214.
CH), 7.15 d (2Harom., J 6.6 Hz), 7.30–7.37 m (3Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 20.0 q (Me, J 4.7 Hz), 21.2 (Me), 54.6 (C1), 122.9 q (CF3, J 269.4 Hz), 123.1, 127.5, 128.1, 129.1, 131.3, 131.4, 133.8, 134.5 q (C3, J 33.9 Hz), 137.0, 137.8, 141.8 q (C2, J 6.3 Hz), 149.8. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −60.61 s (CF3). HRMS (MALDI): C18H16F3 found 289.1199 [M + H]+, calcd 289.1214.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH + 2Harom.), 7.12 d (1Harom., J 7.7 Hz), 7.23–7.30 m (3Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 18.7 (Me), 19.8 q (Me, J 4.8 Hz), 54.5 (C1), 122.9 q (CF3, J 269.4 Hz), 127.3, 128.1, 128.6, 129.0, 129.2, 131.1, 131.9, 133.5 q (C3, J 33.9 Hz), 136.3, 137.0, 143.7 q (C2, J 6.4 Hz), 146.9. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −60.50 s (CF3). HRMS (MALDI): C18H16F3 found 289.1199 [M + H]+, calcd 289.1208.
CH + 2Harom.), 7.12 d (1Harom., J 7.7 Hz), 7.23–7.30 m (3Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 18.7 (Me), 19.8 q (Me, J 4.8 Hz), 54.5 (C1), 122.9 q (CF3, J 269.4 Hz), 127.3, 128.1, 128.6, 129.0, 129.2, 131.1, 131.9, 133.5 q (C3, J 33.9 Hz), 136.3, 137.0, 143.7 q (C2, J 6.4 Hz), 146.9. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −60.50 s (CF3). HRMS (MALDI): C18H16F3 found 289.1199 [M + H]+, calcd 289.1208.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH + 2Harom.), 7.21–7.29 m (3Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 15.5 (Me), 19.5 (Me), 19.7 q (Me, J 4.8 Hz), 54.6 (C1), 122.9 q (CF3, J 269.4 Hz), 126.9, 127.2, 128.0, 128.6, 129.0, 132.7, 133.2 q (C3, J 33.8 Hz), 134.9, 135.7, 137.1, 142.8 q (C2, J 6.4 Hz), 147.2. 19F NMR (CDCl3, 376 MHz) δ, ppm: −60.69 d (CF3, J 1.8 Hz). HRMS (MALDI): C19H18F3 found 303.1355 [M + H]+, calcd 303.1361.
CH + 2Harom.), 7.21–7.29 m (3Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 15.5 (Me), 19.5 (Me), 19.7 q (Me, J 4.8 Hz), 54.6 (C1), 122.9 q (CF3, J 269.4 Hz), 126.9, 127.2, 128.0, 128.6, 129.0, 132.7, 133.2 q (C3, J 33.8 Hz), 134.9, 135.7, 137.1, 142.8 q (C2, J 6.4 Hz), 147.2. 19F NMR (CDCl3, 376 MHz) δ, ppm: −60.69 d (CF3, J 1.8 Hz). HRMS (MALDI): C19H18F3 found 303.1355 [M + H]+, calcd 303.1361.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.27–7.31 m (2Harom.), 7.36 dt (1Harom., J 7.2 Hz, J 1.8 Hz), 7.53 dd (1Harom., J 7.5 Hz, J 0.8 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 55.2 (C1), 101.3, 108.0, 108.7, 121.0, 121.4, 122.5 q (CF3, J 270.0 Hz), 124.5, 127.0, 127.5, 134.5 q (C3, J 34.2 Hz), 138.1, 141.1 q (C2, J 5.0 Hz), 147.1, 148.1, 148.2. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.07 s (CF3). HRMS (MALDI): C17H12F3O2 found 305.0784 [M + H]+, calcd 305.0792.
CH), 7.27–7.31 m (2Harom.), 7.36 dt (1Harom., J 7.2 Hz, J 1.8 Hz), 7.53 dd (1Harom., J 7.5 Hz, J 0.8 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 55.2 (C1), 101.3, 108.0, 108.7, 121.0, 121.4, 122.5 q (CF3, J 270.0 Hz), 124.5, 127.0, 127.5, 134.5 q (C3, J 34.2 Hz), 138.1, 141.1 q (C2, J 5.0 Hz), 147.1, 148.1, 148.2. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.07 s (CF3). HRMS (MALDI): C17H12F3O2 found 305.0784 [M + H]+, calcd 305.0792.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.05 d (2Harom., J 7.7 Hz), 7.28–7.30 m (4Harom.), 7.37–7.43 m (1Harom.), 7.59 d (1Harom., J 6.9 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 54.9 (C1), 121.1, 122.4 q (CF3, J 270.0 Hz), 124.5, 127.2, 127.7, 129.3, 129.4, 133.5, 135.0 q (C3, J 34.4 Hz), 135.6, 138.1, 140.5 q (C2, J 4.9 Hz), 147.6. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.04 s (CF3). HRMS (MALDI): C16H11F335Cl found 295.0496 [M + H]+, calcd 295.0503.
CH), 7.05 d (2Harom., J 7.7 Hz), 7.28–7.30 m (4Harom.), 7.37–7.43 m (1Harom.), 7.59 d (1Harom., J 6.9 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 54.9 (C1), 121.1, 122.4 q (CF3, J 270.0 Hz), 124.5, 127.2, 127.7, 129.3, 129.4, 133.5, 135.0 q (C3, J 34.4 Hz), 135.6, 138.1, 140.5 q (C2, J 4.9 Hz), 147.6. 19F {1H} NMR (CDCl3, 376 MHz) δ, ppm: −64.04 s (CF3). HRMS (MALDI): C16H11F335Cl found 295.0496 [M + H]+, calcd 295.0503.
Synthesis and characterization of indenes 4
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 2.0 Hz), 7.32 t (1Harom., J 7.5 Hz), 7.40–7.50 m (4Harom.), 7.55–7.62 m (3Harom.), 7.65 d (1Harom., J 7.5 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 52.7 q (C1, J 29.4 Hz), 121.3, 124.8 q (C2, J 2.7 Hz), 124.9, 126.3 q (CF3, J 278.5 Hz), 126.4, 127.8, 128.5, 128.8, 129.5, 134.6, 138.8, 144.2, 149.4. 19F NMR (CDCl3, 376 MHz) δ, ppm: −67.35 dd (CF3, J 9.3 Hz, J 0.7 Hz). HRMS (MALDI): C16H12F3 found 261.0886 [M + H]+, calcd 261.0885.
CH, J 2.0 Hz), 7.32 t (1Harom., J 7.5 Hz), 7.40–7.50 m (4Harom.), 7.55–7.62 m (3Harom.), 7.65 d (1Harom., J 7.5 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 52.7 q (C1, J 29.4 Hz), 121.3, 124.8 q (C2, J 2.7 Hz), 124.9, 126.3 q (CF3, J 278.5 Hz), 126.4, 127.8, 128.5, 128.8, 129.5, 134.6, 138.8, 144.2, 149.4. 19F NMR (CDCl3, 376 MHz) δ, ppm: −67.35 dd (CF3, J 9.3 Hz, J 0.7 Hz). HRMS (MALDI): C16H12F3 found 261.0886 [M + H]+, calcd 261.0885.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 2.0 Hz), 7.30 d (2Harom., J 7.9 Hz), 7.34 t (1Harom., J 7.5 Hz), 7.43 t (1Harom., J 7.5 Hz), 7.52 d (2Harom., J 7.9 Hz), 7.58 d (1Harom., J 7.5 Hz), 7.68 d (1Harom., J 7.5 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.6 (Me), 52.7 q (C1, J 29.5 Hz), 121.3, 124.2 q (C2, J 2.7 Hz), 124.9, 126.3 q (CF3, J 278.5 Hz), 126.4, 127.7, 128.5, 129.5, 131.8, 138.5, 138.9 q (J 1.6 Hz), 144.4, 149.3. 19F NMR (CDCl3, 376 MHz) δ, ppm: −67.31 d (CF3, J 9.3 Hz). HRMS (MALDI): C17H14F3 found 275.1042 [M + H]+, calcd 275.1047.
CH, J 2.0 Hz), 7.30 d (2Harom., J 7.9 Hz), 7.34 t (1Harom., J 7.5 Hz), 7.43 t (1Harom., J 7.5 Hz), 7.52 d (2Harom., J 7.9 Hz), 7.58 d (1Harom., J 7.5 Hz), 7.68 d (1Harom., J 7.5 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.6 (Me), 52.7 q (C1, J 29.5 Hz), 121.3, 124.2 q (C2, J 2.7 Hz), 124.9, 126.3 q (CF3, J 278.5 Hz), 126.4, 127.7, 128.5, 129.5, 131.8, 138.5, 138.9 q (J 1.6 Hz), 144.4, 149.3. 19F NMR (CDCl3, 376 MHz) δ, ppm: −67.31 d (CF3, J 9.3 Hz). HRMS (MALDI): C17H14F3 found 275.1042 [M + H]+, calcd 275.1047.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 2.1 Hz), 7.16 d (1Harom., J 7.6 Hz), 7.30 d (2Harom., J 7.9 Hz), 7.38 s (2Harom.), 7.51 d (2Harom., J 7.9 Hz), 7.55 d (1Harom., J 7.6 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.4 (Me), 21.8 (Me), 52.4 (C1, J 29.4 Hz), 122.1, 124.5 q (C2, J 2.8 Hz), 124.6, 126.4 q (CF3, J 278.4 Hz), 127.1, 127.8, 129.5, 131.9, 136.0 q (J 1.8 Hz), 138.4, 138.5, 144.6, 149.3. 19F NMR (CDCl3, 376 MHz) δ, ppm: −67.49 d (CF3, J 9.4 Hz). HRMS (MALDI): C18H16F3 found 289.1199 [M + H]+, calcd 289.1209.
CH, J 2.1 Hz), 7.16 d (1Harom., J 7.6 Hz), 7.30 d (2Harom., J 7.9 Hz), 7.38 s (2Harom.), 7.51 d (2Harom., J 7.9 Hz), 7.55 d (1Harom., J 7.6 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.4 (Me), 21.8 (Me), 52.4 (C1, J 29.4 Hz), 122.1, 124.5 q (C2, J 2.8 Hz), 124.6, 126.4 q (CF3, J 278.4 Hz), 127.1, 127.8, 129.5, 131.9, 136.0 q (J 1.8 Hz), 138.4, 138.5, 144.6, 149.3. 19F NMR (CDCl3, 376 MHz) δ, ppm: −67.49 d (CF3, J 9.4 Hz). HRMS (MALDI): C18H16F3 found 289.1199 [M + H]+, calcd 289.1209.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 2.1 Hz), 7.01 d (2Harom., J 8.8 Hz), 7.33 dt (1Harom., J 7.4 Hz, J 0.9 Hz), 7.42 t (1Harom., J 7.4 Hz), 7.54–7.58 m (3Harom.), 7.66 d (1Harom., J 7.4 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 52.7 q (C1, J 29.4 Hz), 55.5 (OMe), 114.3, 121.3, 123.7 q (C2, J 2.8 Hz), 124.9, 126.3 q (CF3, J 278.5 Hz), 127.2, 128.5, 129.1, 138.9, 144.5, 148.9, 160.0. 19F NMR (CDCl3, 376 MHz) δ, ppm: −67.34 d (CF3, J 9.5 Hz). HRMS (MALDI): C17H14F3O found 291.0991 [M + H]+, calcd 291.0990.
CH, J 2.1 Hz), 7.01 d (2Harom., J 8.8 Hz), 7.33 dt (1Harom., J 7.4 Hz, J 0.9 Hz), 7.42 t (1Harom., J 7.4 Hz), 7.54–7.58 m (3Harom.), 7.66 d (1Harom., J 7.4 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 52.7 q (C1, J 29.4 Hz), 55.5 (OMe), 114.3, 121.3, 123.7 q (C2, J 2.8 Hz), 124.9, 126.3 q (CF3, J 278.5 Hz), 127.2, 128.5, 129.1, 138.9, 144.5, 148.9, 160.0. 19F NMR (CDCl3, 376 MHz) δ, ppm: −67.34 d (CF3, J 9.5 Hz). HRMS (MALDI): C17H14F3O found 291.0991 [M + H]+, calcd 291.0990.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 1.9), 7.07 c (1Harom.), 7.22 c (1Harom.), 7.43 t (1Harom., J 7.3 Hz), 7.49 t (2Harom., J 7.3 Hz), 7.59 d (2Harom., J 7.3 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 52.5 q (C1, J 29.4 Hz), 56.3 (OMe), 56.5 (OMe), 104.9, 108.8, 123.5 q (C2, J 2.8 Hz), 126.3 q (CF3, J 278.5 Hz), 127.7, 128.6, 128.9, 131.3, 135.0, 137.3, 148.4, 149.2, 149.8. 19F NMR (CDCl3, 376 MHz) δ, ppm: −67.58 d (CF3, J 9.3 Hz). HRMS (MALDI): C18H16F3O2 found 321.1097 [M + H]+, calcd 321.1097.
CH, J 1.9), 7.07 c (1Harom.), 7.22 c (1Harom.), 7.43 t (1Harom., J 7.3 Hz), 7.49 t (2Harom., J 7.3 Hz), 7.59 d (2Harom., J 7.3 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 52.5 q (C1, J 29.4 Hz), 56.3 (OMe), 56.5 (OMe), 104.9, 108.8, 123.5 q (C2, J 2.8 Hz), 126.3 q (CF3, J 278.5 Hz), 127.7, 128.6, 128.9, 131.3, 135.0, 137.3, 148.4, 149.2, 149.8. 19F NMR (CDCl3, 376 MHz) δ, ppm: −67.58 d (CF3, J 9.3 Hz). HRMS (MALDI): C18H16F3O2 found 321.1097 [M + H]+, calcd 321.1097.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 1.9 Hz), 6.98 d (1Harom., J 8.2 Hz), 7.09 d (1Harom., J 1.9 Hz), 7.14–7.19 m (2Harom.), 7.38 c (1Harom.), 7.54 d (1Harom., J 7.6 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.8 (Me), 52.3 q (C1, J 29.5 Hz), 56.1 (OMe), 56.2 (OMe), 111.2, 111.4, 120.4, 122.0, 124.2 q (C2, J 2.7 Hz), 124.6, 126.3 q (CF3, J 278.4 Hz), 127.2, 127.6, 136.0 d (J 1.9 Hz), 138.5, 144.6, 149.1, 149.3 149.5. 19F NMR (CDCl3, 376 MHz) δ, ppm: −67.48 d (CF3, J 9.3 Hz). HRMS (MALDI): C19H18F3O2 found 335.1253 [M + H]+, calcd 335.1258.
CH, J 1.9 Hz), 6.98 d (1Harom., J 8.2 Hz), 7.09 d (1Harom., J 1.9 Hz), 7.14–7.19 m (2Harom.), 7.38 c (1Harom.), 7.54 d (1Harom., J 7.6 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 21.8 (Me), 52.3 q (C1, J 29.5 Hz), 56.1 (OMe), 56.2 (OMe), 111.2, 111.4, 120.4, 122.0, 124.2 q (C2, J 2.7 Hz), 124.6, 126.3 q (CF3, J 278.4 Hz), 127.2, 127.6, 136.0 d (J 1.9 Hz), 138.5, 144.6, 149.1, 149.3 149.5. 19F NMR (CDCl3, 376 MHz) δ, ppm: −67.48 d (CF3, J 9.3 Hz). HRMS (MALDI): C19H18F3O2 found 335.1253 [M + H]+, calcd 335.1258.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 2.0 Hz), 7.27–7.31 m (1Harom.), 7.40–7.50 m (4Harom.), 7.60 d (2Harom., J 8.4 Hz). 13C NMR (CDCl3, 100 MHz) (from the spectrum of a mixture of isomers, some signals) δ, ppm: 20.1 (Me), 20.3 (Me), 55.4 q (C1, J 29.4 Hz), 124.0 q (C2, J 2.6 Hz), 126.4 q (CF3, J 278.5 Hz). 19F NMR (CDCl3, 376 MHz) (from the spectrum of a mixture of isomers) δ, ppm: −67.45 d (CF3, J 9.0 Hz). Mass spectrum (GC-MS), m/z (IotH., %): 288 [M]+ (100). HRMS (MALDI): C18H16F3 found 289.1199 [M + H]+, calcd 289.1202 (for a mixture of isomers).
CH, J 2.0 Hz), 7.27–7.31 m (1Harom.), 7.40–7.50 m (4Harom.), 7.60 d (2Harom., J 8.4 Hz). 13C NMR (CDCl3, 100 MHz) (from the spectrum of a mixture of isomers, some signals) δ, ppm: 20.1 (Me), 20.3 (Me), 55.4 q (C1, J 29.4 Hz), 124.0 q (C2, J 2.6 Hz), 126.4 q (CF3, J 278.5 Hz). 19F NMR (CDCl3, 376 MHz) (from the spectrum of a mixture of isomers) δ, ppm: −67.45 d (CF3, J 9.0 Hz). Mass spectrum (GC-MS), m/z (IotH., %): 288 [M]+ (100). HRMS (MALDI): C18H16F3 found 289.1199 [M + H]+, calcd 289.1202 (for a mixture of isomers).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 2.1 Hz), 6.98 c (1Harom.), 7.18 c (1Harom.), 7.41–7.50 m (3Harom.), 7.57–7.59 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 20.0 q (Me, J 3.7 Hz), 21.5 (Me), 52.0 q (C1, J 29.2 Hz), 119.9, 125.9 q (C2, J 3.1 Hz), 126.7 q (CF3, J 279.9 Hz), 128.0, 128.5, 128.8, 129.7, 134.2, 135.0, 135.2, 138.7, 145.3, 149.4. 19F NMR (CDCl3, 376 MHz) δ, ppm: −63.90 dd (CF3, J 8.2 Hz, J 1.3 Hz). HRMS (MALDI): C18H16F3 found 289.1199 [M + H]+, calcd 289.1207.
CH, J 2.1 Hz), 6.98 c (1Harom.), 7.18 c (1Harom.), 7.41–7.50 m (3Harom.), 7.57–7.59 m (2Harom.). 13C NMR (CDCl3, 100 MHz) δ, ppm: 20.0 q (Me, J 3.7 Hz), 21.5 (Me), 52.0 q (C1, J 29.2 Hz), 119.9, 125.9 q (C2, J 3.1 Hz), 126.7 q (CF3, J 279.9 Hz), 128.0, 128.5, 128.8, 129.7, 134.2, 135.0, 135.2, 138.7, 145.3, 149.4. 19F NMR (CDCl3, 376 MHz) δ, ppm: −63.90 dd (CF3, J 8.2 Hz, J 1.3 Hz). HRMS (MALDI): C18H16F3 found 289.1199 [M + H]+, calcd 289.1207.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 2.1 Hz), 7.35 dt (1Harom., J 7.4 Hz, J 0.9 Hz), 7.41–7.46 m (3Harom.), 7.50–7.55 m (3Harom.), 7.67 d (1Harom., J 7.4 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 52.8 q (C1, J 29.6 Hz), 121.1, 125.1, 125.2 q (C2, J 2.8 Hz), 126.1 q (CF3, J 278.6 Hz), 126.7, 128.7, 129.1, 129.2, 133.1, 134.6, 138.7, 143.9, 148.4. 19F NMR (CDCl3, 376 MHz) δ, ppm: −67.23 d (CF3, J 9.3 Hz). HRMS (MALDI): C16H11F3Cl found 295.0496 [M + H]+, calcd 295.0496.
CH, J 2.1 Hz), 7.35 dt (1Harom., J 7.4 Hz, J 0.9 Hz), 7.41–7.46 m (3Harom.), 7.50–7.55 m (3Harom.), 7.67 d (1Harom., J 7.4 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 52.8 q (C1, J 29.6 Hz), 121.1, 125.1, 125.2 q (C2, J 2.8 Hz), 126.1 q (CF3, J 278.6 Hz), 126.7, 128.7, 129.1, 129.2, 133.1, 134.6, 138.7, 143.9, 148.4. 19F NMR (CDCl3, 376 MHz) δ, ppm: −67.23 d (CF3, J 9.3 Hz). HRMS (MALDI): C16H11F3Cl found 295.0496 [M + H]+, calcd 295.0496.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.03 dt (1Harom., J 8.5 Hz, J 2.2 Hz), 7.25–7.35 m (3Harom.), 7.37 c (1Harom.), 7.60 dd (1Harom., J 7.7 Hz, J 5.4 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 19.7 (Me), 20.0 (Me), 52.2 q (C1, J 29.7 Hz), 109.0 d (J 24.2 Hz), 113.0 d (J 23.2 Hz), 125.1, 125.8 d (J 9.3 Hz), 126.0 q (C2, J 2.6 Hz), 126.1 q (CF3, J 278.5 Hz), 128.9, 130.2, 131.6, 134.2 m, 137.4 d (J 25.7 Hz), 146.8 d (J 9.0 Hz), 148.8 d (J 2.9 Hz), 163.6 d (J 245.3 Hz). 19F NMR (CDCl3, 376 MHz) δ, ppm: −113.55 to −113.48 m (1Farom.), −67.52 d (CF3, J 9.1 Hz). HRMS (MALDI): C18H15F4 found 307.1104 [M + H]+, calcd 307.1107.
CH), 7.03 dt (1Harom., J 8.5 Hz, J 2.2 Hz), 7.25–7.35 m (3Harom.), 7.37 c (1Harom.), 7.60 dd (1Harom., J 7.7 Hz, J 5.4 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 19.7 (Me), 20.0 (Me), 52.2 q (C1, J 29.7 Hz), 109.0 d (J 24.2 Hz), 113.0 d (J 23.2 Hz), 125.1, 125.8 d (J 9.3 Hz), 126.0 q (C2, J 2.6 Hz), 126.1 q (CF3, J 278.5 Hz), 128.9, 130.2, 131.6, 134.2 m, 137.4 d (J 25.7 Hz), 146.8 d (J 9.0 Hz), 148.8 d (J 2.9 Hz), 163.6 d (J 245.3 Hz). 19F NMR (CDCl3, 376 MHz) δ, ppm: −113.55 to −113.48 m (1Farom.), −67.52 d (CF3, J 9.1 Hz). HRMS (MALDI): C18H15F4 found 307.1104 [M + H]+, calcd 307.1107.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH, J 2.1 Hz), 6.97 d (1Harom., J 8.2 Hz), 7.02 dt (1Harom., J 8.7 Hz, J 2.4 Hz), 7.06 d (1Harom., J 1.9 Hz), 7.14 dd (1Harom., J 8.2 Hz, J 1.9 Hz), 7.26 dd (1Harom., J 9.0 Hz, J 2.4 Hz), 7.58 dd (Harom., J 8.0 Hz, J 5.1 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 52.2 q (C1, J 29.8 Hz), 56.1 (OMe), 56.2 (OMe), 108.9 d (J 24.3 Hz), 110.9, 111.5, 113.1 d (J 23.2 Hz), 120.3, 125.8 q (C2, J 2.6 Hz), 125.9 d (J 9.2 Hz), 126.0 q (CF3, J 278.5 Hz), 126.8, 134.1 m, 146.7 d (J 8.8 Hz), 148.5 d (J 2.9 Hz), 149.6 d (J 44.1 Hz), 163.6 d (J 245.5 Hz). 19F NMR (CDCl3, 376 MHz) δ, ppm: −113.31 dt (1Farom., J 9.0 Hz, J 5.1 Hz), −67.51 d (CF3, J 8.9 Hz). HRMS (MALDI): C18H15F4O2 found 339.1003 [M + H]+, calcd 339.1009.
CH, J 2.1 Hz), 6.97 d (1Harom., J 8.2 Hz), 7.02 dt (1Harom., J 8.7 Hz, J 2.4 Hz), 7.06 d (1Harom., J 1.9 Hz), 7.14 dd (1Harom., J 8.2 Hz, J 1.9 Hz), 7.26 dd (1Harom., J 9.0 Hz, J 2.4 Hz), 7.58 dd (Harom., J 8.0 Hz, J 5.1 Hz). 13C NMR (CDCl3, 100 MHz) δ, ppm: 52.2 q (C1, J 29.8 Hz), 56.1 (OMe), 56.2 (OMe), 108.9 d (J 24.3 Hz), 110.9, 111.5, 113.1 d (J 23.2 Hz), 120.3, 125.8 q (C2, J 2.6 Hz), 125.9 d (J 9.2 Hz), 126.0 q (CF3, J 278.5 Hz), 126.8, 134.1 m, 146.7 d (J 8.8 Hz), 148.5 d (J 2.9 Hz), 149.6 d (J 44.1 Hz), 163.6 d (J 245.5 Hz). 19F NMR (CDCl3, 376 MHz) δ, ppm: −113.31 dt (1Farom., J 9.0 Hz, J 5.1 Hz), −67.51 d (CF3, J 8.9 Hz). HRMS (MALDI): C18H15F4O2 found 339.1003 [M + H]+, calcd 339.1009.
Acknowledgements
This work was supported by the Russian Scientific Foundation (grant no. 14-13-00448). Spectral studies were performed at the Center for Magnetic Resonance, and the Center for Chemical Analysis and Materials Research of Saint Petersburg State University, Saint Petersburg, Russia.References
- A. N. Kazakova, R. O. Iakovenko, V. M. Muzalevskiy, I. A. Boyarskaya, M. S. Avdontceva, G. L. Starova, A. V. Vasilyev and V. G. Nenajdenko, Tetrahedron Lett., 2014, 55, 6851–6855 CrossRef CAS.
- A. N. Kazakova, R. O. Iakovenko, I. A. Boyarskaya, V. G. Nenajdenko and A. V. Vasilyev, J. Org. Chem., 2015, 80, 9506–9517 CrossRef CAS PubMed.
- A. N. Kazakova, R. O. Iakovenko, I. A. Boyarskaya, A. Yu. Ivanov, M. S. Avdontceva, A. A. Zolotarev, T. L. Panikorovsky, G. L. Starova, V. G. Nenajdenko and A. V. Vasilyev, Org. Chem. Front., 2017, 4, 255–265 RSC.
- B. Gabriele, R. Mancuso and L. Veltri, Chem. – Eur. J., 2016, 22, 5056–5094 CrossRef CAS PubMed.
- N. B. Ivchenko, P. V. Ivchenko and I. E. Nifantev, Russ. J. Org. Chem., 2000, 36, 609–637 CAS.
- D. Kuck, Chem. Rev., 2006, 106, 4885–4925 CrossRef CAS PubMed.
- M. Koca, K. O. Yerdelen, B. Anil, Z. Kasap, H. Sevindik, I. Ozyurek, G. Gunesacar and K. Turkaydin, J. Enzyme Inhib. Med. Chem., 2016, 31, 13–23 CrossRef PubMed.
- Z. Liu, R. Zhang, Q. Meng, X. Zhang and Y. Sun, Med. Chem. Commun., 2016, 7, 1352–1355 RSC.
- R. Leino, P. Lehmus and A. Lehtonen, Eur. J. Inorg. Chem., 2004, 3201–3222 CrossRef CAS.
- V. Cadierno, J. Díez, M. P. Gamasa, J. Gimeno and E. Lastra, Coord. Chem. Rev., 1999, 193–195, 147–205 CrossRef CAS.
- D. Zargarian, Coord. Chem. Rev., 2002, 233–234, 157–176 CrossRef CAS.
- C. Sui-Seng, A. Castonguay, Y. Chen, D. Gareau, L. F. Groux and D. Zargarian, Top. Catal., 2006, 37, 81–90 CrossRef CAS.
- S. Radix-Large, S. Kucharski and B. R. Langlois, Synthesis, 2004, 456–465 CAS.
- A. Boreux, G. H. Lonca, O. Riant and F. Gagosz, Org. Lett., 2016, 18, 5162–5165 CrossRef CAS PubMed.
- N. Ghavtadze, F. Roland and E.-U. Wuerthwein, J. Org. Chem., 2009, 74, 4584–4591 CrossRef CAS PubMed.
- A. D. Allen, M. Fujio, N. Mohammed, T. Tidwell and Y. Tsuji, J. Org. Chem., 1997, 62, 246–252 CrossRef CAS PubMed.
- P. G. Gassman, J. A. Ray, P. G. Wenthold and J. W. Mickelson, J. Org. Chem., 1991, 56, 5143–5146 CrossRef CAS.
- R. G. Parr, L. v. Szentpaly and S. Liu, J. Am. Chem. Soc., 1999, 121, 1922–1924 CrossRef CAS.
- P. K. Chattaraj, S. Giri and S. Duley, Chem. Rev., 2011, 111, PR43–PR75 CrossRef PubMed.
- C. D. Smith, G. Rosocha, L. Mui and R. A. Batey, J. Org. Chem., 2010, 75, 4716–4727 CrossRef CAS PubMed.
- M. Alajarin, M. Marin-Luna, P. Sanchez-Andrada and A. Vidal, Beilstein J. Org. Chem., 2016, 12, 260–270 CrossRef CAS PubMed.
- J. Cipot, D. Wechsler, M. Stradiotto, R. McDonald and M. J. Ferguson, Organometallics, 2003, 22, 5185–5192 CrossRef CAS.
- P. E. Rakita and G. A. Taylor, J. Organomet. Chem., 1973, 61, 71–81 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford, CT, 2010 Search PubMed.
- S. Large, N. Roques and B. R. Langlois, J. Org. Chem., 2000, 65, 8848–8856 CrossRef CAS PubMed.
- R. P. Singh, R. L. Kirchmeier and J. M. Shreeve, Org. Lett., 1999, 1, 1047–1049 CrossRef CAS.
- A. D. Cort, Synth. Commun., 1990, 20, 757–760 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C, 19F NMR spectra of compounds, and data on DFT calculations. See DOI: 10.1039/c7ob00406k |
| This journal is © The Royal Society of Chemistry 2017 |