Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
JA-Ile-macrolactones uncouple growth and defense in wild tobacco†
Guillermo H.
Jimenez-Aleman
*a,
Ricardo A. R.
Machado‡
b,
Ian T.
Baldwin
b and
Wilhelm
Boland
 *a
*a
aDepartment of Bioorganic Chemistry, Max Planck Institute für chemische Ökologie, 07745 Jena, Germany. E-mail: gjimenez-aleman@ice.mpg.de; boland@ice.mpg.de
bDepartment of Molecular Ecology, Max Planck Institute für chemische Ökologie, 07745 Jena, Germany
First published on 28th February 2017
Abstract
Small molecules capable of uncoupling growth-defense in plants are currently not known. In this study, for the first time, semi-synthetic analogues of the phytohormone JA-Ile are employed to uncouple growth and defense responses in wild tobacco. The JA-Ile analogues are easily synthesized from inexpensive substrates via olefin metathesis.
Plant growth is often compromised when the plant immune system is activated in response to environmental challenges such as pathogens and herbivores.1 These negative associations between growth and defense are, to an important extent, controlled by phytohormones such as jasmonates (Scheme 1).1–3N-Jasmonoyl-L-isoleucine (JA-Ile, 4) is the most active endogenous jasmonate. This molecule mediates several processes such as development of reproductive organs4 and activation of plant defenses against necrotrophic pathogens and herbivores.5,6 These properties of JA-Ile (4) could be exploited in agricultural systems, but the activation of the jasmonate signaling (JA-signaling) often results in the reduction of plant growth and fitness, drawbacks which restrain the use of bioactive jasmonates for crop protection.7,8 In a recent study, genetic manipulation of the JA-signaling was used to uncouple growth and defense in Arabidopsis thaliana.3 The loss-of-function mutants did not have five of the thirteen JASMONATE-ZIM-DOMAIN (JAZ) proteins – which act as co-receptors of JA-Ile (4) in the JA-signaling pathway. Although genetic manipulation is a promising approach in this context, it may be limited by both the lack of molecular tools in many plant species of economic interest and the still strong popular opposition to genetically-modified crops. An alternative to overcome these limitations could be the use of small molecules (e.g. analogs of phytohormones) in order to uncouple growth and defense in wild type crop plants.
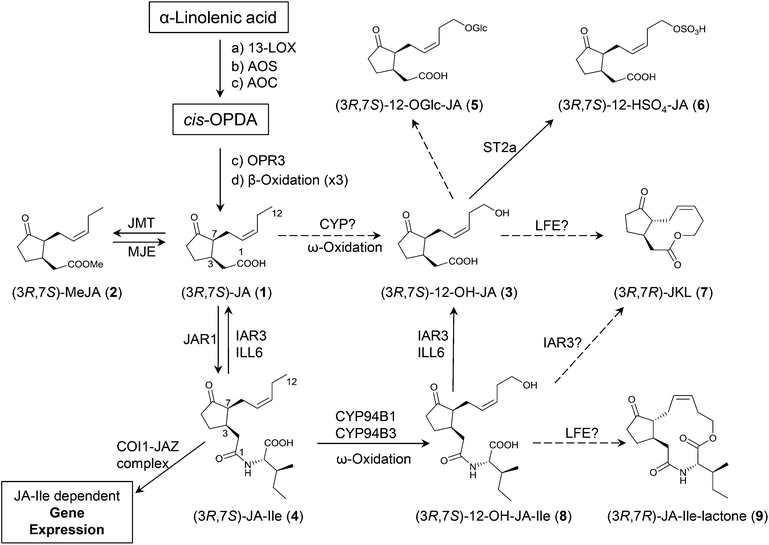 | ||
| Scheme 1 Simplified scheme of jasmonates biosynthesis. 13-LOX (13-Lipoxygenase); AOS (Allene Oxide Synthase); AOC (Allene Oxide Cyclase); OPR3 (12-Oxophytodienoate Reductase 3); JMT (JA Carboxyl Methyltransferase); MJE (Methyljasmonate Esterase); ILL6/IAR3 (JA-Ile Amidohydrolases); CYP94B1/B3/C1 (Cytochromes P450); COI1-JAZ (Coronatine Insensitive 1-JAZ complex, JA-Ile receptor complex); LFE (Lactone Forming Enzyme). A dashed arrow represents a proposed transformation. Nomenclature and numbering commonly used for jasmonates are employed for clarity. Epimerization at C7 may occur: less active 7R epimers exist in nature. For a comprehensive review on jasmonates biosynthesis, signaling and activity please see ref. 5. | ||
Several analogs of JA-Ile (4) have been employed to manipulate the JA-signaling pathway.9–12 Based on this, and on preliminary observations in a previous work with ω-modified jasmonates (macrolactones 9 and 9a, Scheme 2),13 the possibility of uncoupling growth from defense responses by using JA-Ile (4) analogs was explored. Diastereomeric JA-Ile-lactones 9 and 9a are a new class of JA-Ile derivatives capable of inducing nicotine accumulation in Nicotiana attenuata leaves to a similar extent as endogenous bioactive jasmonates do.13 In this study, N. attenuata plants were treated with macrolactones 9 and 9a and the performance of Manduca sexta caterpillars on treated plants, plant growth rates and reproductive output were evaluated. In addition, a new synthetic route (Scheme 2) to JA-Ile-lactones 9 and 9a was developed.
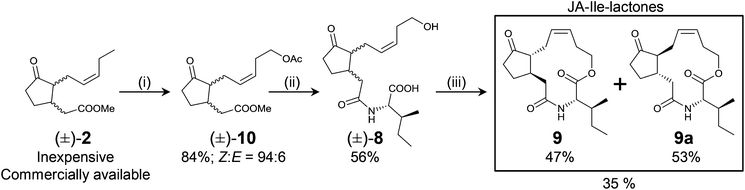 | ||
| Scheme 2 Synthetic route to JA-Ile-lactones. (i) Z-Selective Grubbs type of catalyst (5 mol%), but-3-en-1-yl acetate (6 equiv.), 400 mbar, 45 °C. (ii) 1. Saponification/2. conjugation to L-Ile. (iii) Macrolactonization. Steps (ii) and (iii) as in ref. 13. Employed commercially available (±)-2 was a mixture of all possible diastereoisomers as indicated by the wavy bonds at C3 and C7. Diastereomerically pure macrolactones are obtained after flash-chromatography and recrystallization. | ||
The Z-selective cross-metathesis of (±)-2 and but-3-en-1-yl acetate (both compounds commercially available and inexpensive) afforded compound (±)-10 in excellent yield (>80%) and Z-selectivity (>90%). Saponification of (±)-10 (>85% yield), conjugation of (±)-3 to L-Ile, and macrolactonization of (±)-8 as previously described13 afforded enantiomerically pure macrolactones 9 and 9a in only three linear steps (Scheme 2).
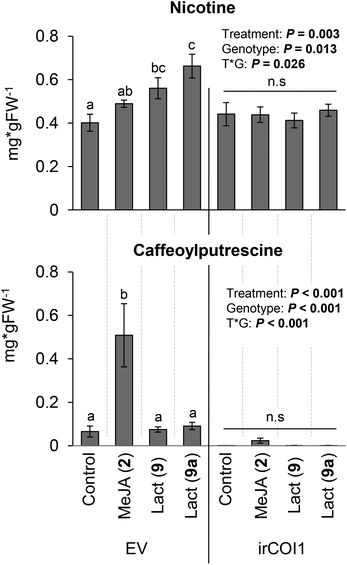
In agreement with our previous study,13 macrolactones 9 and 9a induced the accumulation of nicotine (Fig. 1) – MeJA (2) is converted into bioactive JA-Ile (4) in vivo and was therefore employed as the positive control.14 Moreover, nicotine was not induced in plant genotypes impaired in JA-Ile (4) perception (irCOI1 plants), indicating that JA-Ile-lactones 9 and 9a are perceived by N. attenuata plants in a similar manner as the endogenous bioactive jasmonate JA-Ile (4) (Fig. 1). Interestingly, caffeoylputrescine was induced by MeJA (2) but not by the lactones 9 or 9a (Fig. 1). These outcomes suggest that (i) the lactones can selectively induce nicotine and, perhaps, other plant defenses, (ii) additional elements induced by endogenous jasmonates (4) but not by the lactones 9 or 9a are required for a full induction of jasmonate-dependent defensive secondary metabolites.15
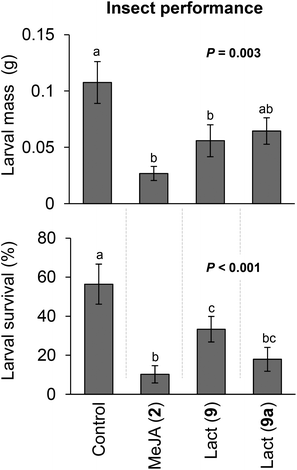
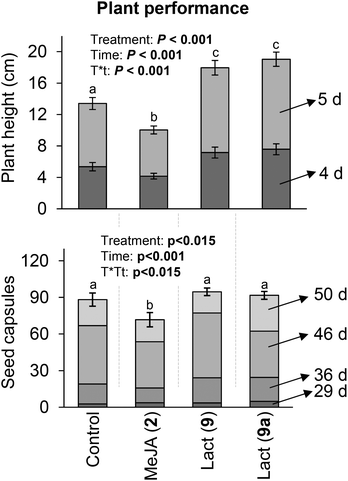
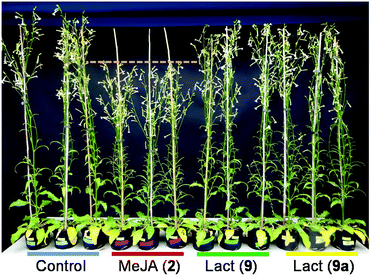
Interestingly, nicotine induced by the lactones was accompanied by a decrease in both M. sexta caterpillar mass gain and survivorship (Fig. 2). Manduca sexta is a specialist insect very tolerant to nicotine, which supports our hypothesis that the lactones activate additional plant defenses apart from nicotine or that nicotine is detrimental for M. sexta at the observed concentrations. Strikingly, contrary to MeJA (2), macrolactones 9 and 9a stimulated plant growth at the early developmental stage and did not affect seed capsule formation (Fig. 3). At the maturing stage, the height of plants treated with the lactones was not different from control plants, but MeJA (2) treated plants were considerably smaller (Fig. 4). These results show that the induction of defenses by the macrolactones 9 or 9a is not accompanied by a decrease in plant growth or fitness as it is generally observed after treatment with endogenous or synthetic jasmonates known to date.1
Our study suggests that N. attenuata plants can simultaneously defend themselves against the attack of the caterpillar and maintain the capability to grow. In N. attenuata, overproduction of jasmonates results in stem stunting due to cross-talk of bioactive jasmonates with other phytohormones.16,17 On the other hand, exogenously applied jasmonates are metabolized in vivo into bioactive jasmonates, resulting in elevated levels of these compounds within plant tissues.18,19 Therefore, it was hypothesized that, contrary to MeJA (2), macrolactones 9 and 9a activate jasmonate-related defenses without increasing the levels of endogenous jasmonates, which results in defended plants with normal growth rates (no interference of endogenous jasmonates with other phytohormones). In agreement with this hypothesis, it was observed that endogenous JA (1) and JA-Ile (4) levels were similar in control and lactone-treated plants, while a significant increase of these compounds was observed in MeJA (2) treated plants (Fig. S1†).
Together these results indicate that JA-Ile-lactones may promote the interaction between the jasmonate receptor COI1 and singular JAZ proteins (repressors of JA-signaling and co-receptors of JA-Ile),20 which results in the observed phenotypes. The recent report on the Arabidopsis thaliana mutant (lacking five of the thirteen JAZs), where growth and defense are uncoupled, supports this hypothesis.3 However, the possibility that JA-Ile-lactones (9) and (9a) exert their action via a non-canonical pathway cannot be ruled out at this point. Likewise, a direct toxicity of lactones 9 and 9a for insects cannot be fully discarded, although their concentration in the leaf tissue is very low (pmoles per gram of fresh weight).
In conclusion, we have developed brief synthetic route based on Z-selective olefin cross-metathesis which allows the preparation of macrolactones 9 and 9a in only three linear steps. The pioneer studies with these compounds open the possibility of uncoupling defense and growth in plants by using small molecules. This idea, a ligand based strategy to activate particular plant responses, might be also applicable to other phytohormones such as gibberellins and brassinosteroids, both occurring in high diversity within plant tissues. Additionally, the presented ligand-based manipulation of the JA-signaling pathway constitutes a promising approach for (i) plant protection that could reduce the use of both pesticides and genetically modified crops, (ii) the use of rationally designed jasmonates as molecular tools to better understand the mechanisms governing growth-defense tradeoffs in plants. The molecular basis underpinning the findings presented here constitute exciting avenues for future research.
Acknowledgements
All experimental work of this study was supported by the Max Planck Society and the DFG (ChemBioSys, CRC 1127). The work of ITB was supported by an Advanced Grant No 293926 of the European Research Council. Special thanks go to Kerstin Ploss for the HRMS measurements, Michael Reichelt for determination of jasmonate levels and the greenhouse team for their support.Notes and references
- B. Huot, J. Yao, B. L. Montgomery and S. Y. He, Mol. Plant, 2014, 7, 1267–1287 CrossRef CAS PubMed.
- S. Gimenez-Ibanez, M. Boter and R. Solano, Essays Biochem., 2015, 58, 83–100 CrossRef PubMed.
- M. L. Campos, Y. Yoshida, I. T. Major, D. de, O. Ferreira, S. M. Weraduwage, J. E. Froehlich, B. F. Johnson, D. M. Kramer, G. Jander, T. D. Sharkey and G. A. Howe, Nat. Commun., 2016, 7, 1–10 Search PubMed.
- T. Qi, H. Huang, S. Song and D. Xie, Plant cell, 2015, 27, 1620–1633 CrossRef CAS PubMed.
- C. Wasternack and B. Hause, Ann. Bot., 2013, 111, 1021–1058 CrossRef CAS PubMed.
- R. A. R. Machado, M. McClure, M. R. Herve, I. T. Baldwin and M. Erb, eLife, 2016, 5, e13720 Search PubMed.
- R. A. R. Machado, A. P. Ferrieri, C. A. Robert, G. Glauser, M. Kallenbach, I. T. Baldwin and M. Erb, New Phytol., 2013, 200, 1234–1246 CrossRef CAS PubMed.
- I. T. Baldwin, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 8113–8118 CrossRef CAS.
- I. Monte, M. Hamberg, A. Chini, S. Gimenez-Ibanez, G. García-Casado, A. Porzel, F. Pazos, M. Boter and R. Solano, Nat. Chem. Biol., 2014, 10, 671–676 CrossRef CAS PubMed.
- R. Lauchli and W. Boland, Chem. Rec., 2003, 3, 12–21 CrossRef CAS PubMed.
- G. Schüler, H. Görls and W. Boland, Eur. J. Org. Chem., 2001, 1663–1668 CrossRef.
- Y. Nakamura, C. Paetz, W. Brandt, A. David, M. Rendon-Anaya, A. Herrera-Estrella, A. Mithöfer and W. Boland, J. Chem. Ecol., 2014, 40, 687–699 CrossRef CAS PubMed.
- G. H. Jimenez-Aleman, R. A. Machado, H. Görls, I. T. Baldwin and W. Boland, Org. Biomol. Chem., 2015, 13, 5885–5893 CAS.
- J. Wu, L. Wang and I. T. Baldwin, Planta, 2008, 227, 1161–1168 CrossRef CAS PubMed.
- R. A. R. Machado, C. A. Robert, C. C. Arce, A. P. Ferrieri, S. Xu, G. H. Jimenez-Aleman, I. T. Baldwin and M. Erb, Plant Physiol., 2016, 172, 521–532 CrossRef CAS PubMed.
- M. Heinrich, C. Hettenhausen, T. Lange, H. Wunsche, J. Fang, I. T. Baldwin and J. Wu, Plant J., 2013, 73, 591–606 CrossRef CAS PubMed.
- R. A. R. Machado, I. T. Baldwin and M. Erb, New Phytol. Search PubMed , submitted.
- G. H. Jimenez-Aleman, S. S. Scholz, M. Heyer, M. Reichelt, A. Mithofer and W. Boland, Biochim. Biophys. Acta, 2015, 1851, 1545–1553 CrossRef CAS PubMed.
- O. Miersch and C. Wasternack, Biol. Chem., 2000, 381, 715–722 CrossRef CAS PubMed.
- L. B. Sheard, X. Tan, H. Mao, J. Withers, G. Ben-Nissan, T. R. Hinds, Y. Kobayashi, F. F. Hsu, M. Sharon, J. Browse, S. Y. He, J. Rizo, G. A. Howe and N. Zheng, Nature, 2010, 468, 400–405 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ob00249a |
| ‡ Present address: Institute of Plant Sciences, Biotic Interactions. University of Bern. Altenbergrain 21, 3013 Bern, Switzerland. |
| This journal is © The Royal Society of Chemistry 2017 |