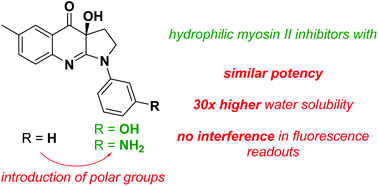
In search of myosin II inhibitors with superior research tool properties, a chemical optimization campaign of the blebbistatin scaffold was conducted in this paper. (S)-Blebbistatin is the best known small-molecule inhibitor of myosin II ATPase activity. Unfortunately, as a research tool this compound has several deficiencies: it is photolabile and (photo)toxic, has low water solubility, and its (fluorescent) precipitates interfere in (fluorescence) readouts. In view of obtaining tool compounds with improved properties, both enantiomers of a series of D-ring modified polar analogs were prepared. We identified (S)-3′-hydroxyblebbistatin (S)-2 and (S)-3′-aminoblebbistatin (S)-3 as two myosin II inhibitors with a 30-fold higher water solubility than (S)-blebbistatin. These molecules furthermore do not cause interference in (fluorescence) readouts. (S)-2 and (S)-3 thus are superior alternatives to (S)-blebbistatin as research tools to study myosin II.