Halogen bonding rotaxanes for nitrate recognition in aqueous media†
Sean W.
Robinson
 and
Paul D.
Beer
*
and
Paul D.
Beer
*
Chemistry Research Laboratory, Department of Chemistry, University of Oxford, 12 Mansfield Road, Oxford, OX1 3TA, UK. E-mail: paul.beer@chem.ox.ac.uk
First published on 16th November 2016
Abstract
Targeting the biologically and environmentally important nitrate anion, halogen bonding (XB) has been incorporated into three novel [2]rotaxane structural frameworks via an axle component containing covalently linked 3,5-bis-iodotriazole pyridine–pyridinium motifs. This has enabled the recognition of nitrate in aqueous media containing up to 90% water with equivalent binding affinity to chloride, illustrating the potency of XB for anion recognition in highly competitive aqueous solvent mixtures.
Introduction
Stimulated by the crucial roles that anions play in chemical, biological and environmental processes, the field of anion recognition has grown dramatically over the last couple of decades with reports of a vast array of synthetic hosts capable of binding anions in organic and aqueous media.1–5 Recently, halogen bonding (XB),6 has been introduced into anion host systems as an alternative to the prevalent hydrogen bond (HB), typically resulting in a dramatic enhancement in anion binding strength as well as noteworthy changes in selectivity.7 Advantageously, XB is comparable in strength to HB, demands strict directionality of interaction, and its pH-independence and solvent resistance provide significant benefits for the binding of anions in aqueous media.8,9 Nonetheless, while anion receptors exploiting XB interactions are relatively rare,10–14 integrating XB motifs into mechanically interlocked anion host systems has facilitated anion recognition in organic–aqueous media and pure water.15–21Of particular biological and environmental importance is the nitrate anion, which has been implicated in blue baby syndrome (methemoglobinemia) and its anthropogenic overuse in arable farming has led to eutrophication of dams and lakes. Various acyclic tripodal systems,22,23 macrocyclic receptors24 and trigonal cages25,26 have been demonstrated to accommodate the oxoanion's trigonal planar geometry, employing spatially-oriented HB amide motifs to complex each of the three nitrate oxygen atoms; however, all these host systems only function in organic solvents.27 Indeed, receptors capable of nitrate recognition in aqueous media are exceptionally scarce. To the best of our knowledge, two [2]rotaxanes and a [2]catenane host containing respectively, a bis-triazolium acridine axle component, and covalently linked isophthalamide-3,5-bis-amide pyridinium axle and macrocycle motifs are the only examples, binding nitrate in aqueous 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CDCl3
10 CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD
CD3OD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O solution.28–30
D2O solution.28–30
In an effort to exploit the stringent directionality of XB as a means of elevating the strength and selectivity of nitrate anion recognition in higher percentage water containing solvent media and taking into account our previous nitrate selective interlocked HB host design, herein we report the synthesis of three XB [2]rotaxane anion receptors which incorporate two XB 3,5-bis-iodotriazole pyridine or pyridinium donor sites into the axle component, one of which is demonstrated to bind nitrate in 90% water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 D2O
1 D2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone-d6).
acetone-d6).
Results and discussion
Synthesis
The synthesis of the crucial XB axle component of the target [2]rotaxane required the incorporation of two XB 3,5-bis-iodotriazole pyridine or pyridinium donor sites while the macrocycle component was prepared via standard procedures.31 Initially, attempts were made to prepare unsymmetrically functionalised axle precursors via various alkyne functional group protection methods. Sonogashira reaction32,33 between commercially available 3,5-dibromo-pyridine and 2-methylbut-3-yn-2-ol afforded 1 in 79% yield. Subsequent Sonogashira reaction32,33 with TBDMS-acetylene gave the unsymmetrically protected 3,5-diethynyl pyridine 2 in 87% yield. However, selective deprotection of 2 with 2-hydroxy propane and TBDMS protecting groups was problematic. Whilst the 2-hydroxy propyl protecting group could easily be selectively cleaved in the presence of TBDMS by refluxing 2 with NaOH in toluene, the use of TBAF to remove TBDMS either resulted in the unexpected removal of the 2-hydroxy propyl protecting group instead (2 to 3) or the decomposition of the iodotriazole group, for example, in 5 to a prototriazole in later steps in the synthesis (Scheme 1).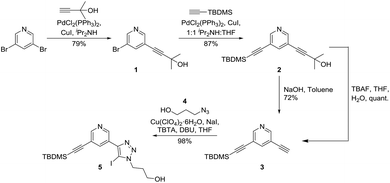 | ||
| Scheme 1 Stepwise alkyne protection and deprotection steps using TBDMS and 2-hydroxy propyl protecting groups. | ||
Since the TMS protecting group offered a more labile alternative to TBDMS, and could be selectively deprotected in the presence of the 2-hydroxy propyl protecting group, the TMS analogue of 2 was prepared using an initial Sonogashira reaction32,34 between 3,5-dibromopyridine and TMS-acetylene to afford 6 in 79% yield. Thereafter, Sonogashira reaction32 with 2-methylbut-3-yn-2-ol gave 7 in 91% yield. The TMS protecting group was selectively deprotected using KOH in MeOH, quantitatively giving 8, which was reacted with various stopper azides (terphenyl-propyl 9, terphenyl-aryl 10 and permethylated β-cyclodextrin 11) using a one-pot iodo-CuAAC click reaction.35 Unfortunately, the click reactions to stopper the mono-deprotected alkyne were unsuccessful (Scheme 2) with the recovery of only starting materials after purification.
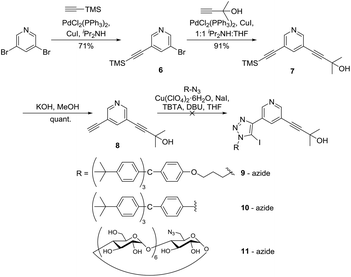 | ||
| Scheme 2 Selective alkyne protection and deprotection steps using TMS and 2-hydroxy propyl protecting groups. | ||
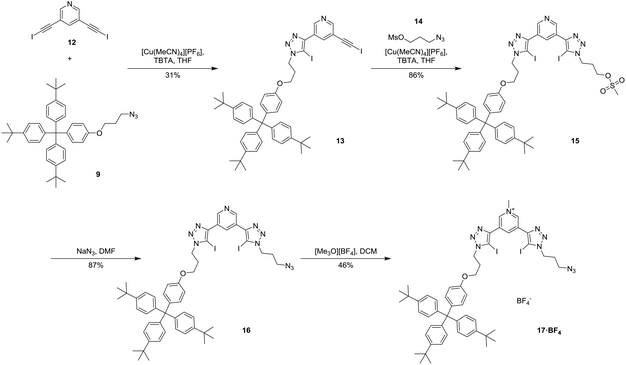
Since stepwise alkyne protection/deprotection synthetic techniques could not be used to prepare the axle component, the synthesis of target XB [2]rotaxanes 19·PF6 and 20·(PF6)2 was reliant upon an initial statistical stoppering step shown in Scheme 3. Employing a modification of the copper(I)-catalysed azide–iodoalkyne cycloaddition (CuAAC) click reaction,36,37 an equimolar mixture of 3,5-diiodoethynyl pyridine 12 and terphenyl stopper azide 9 in the presence of Cu(I) and TBTA in THF afforded the mono-substituted precursor 13 in 31% yield. Reaction of azido-propyl-mesylate 14 with 13 using the same CuAAC conditions gave the intermediate 15 in 86% yield. Substitution of the mesylate to produce azide 16 was achieved with NaN3 in 87% yield and methylation with [Me3O][BF4] gave the pyridinium tetrafluoroborate salt 17·BF4 in 46% yield.
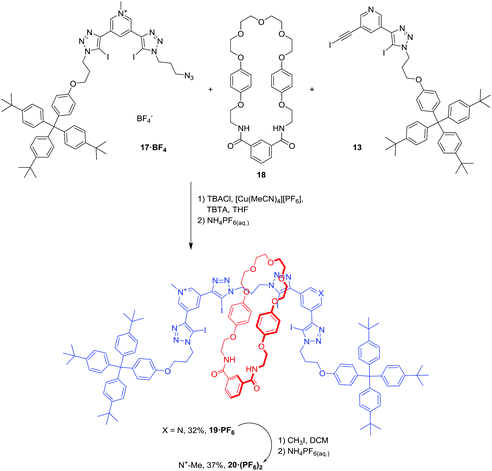
The synthesis of rotaxanes 19·PF6 and 20·(PF6)2 is shown in Scheme 4. The synthesis of [2]rotaxane 19·PF6 was achieved in 32% yield using a chloride anion-templated mono-stoppering CuAAC click methodology: equimolar amounts of 13 and 17·BF4 were mixed with 10 equivalents of isophthalamide macrocycle 18 in the presence of Cu(I), TBTA and one equivalent of TBA·Cl. Methylation of [2]rotaxane 19·PF6 with CH3I afforded the dicationic [2]rotaxane 20·(PF6)2 in 37% yield after anion exchange to the non-coordinating hexafluorophosphate anion.
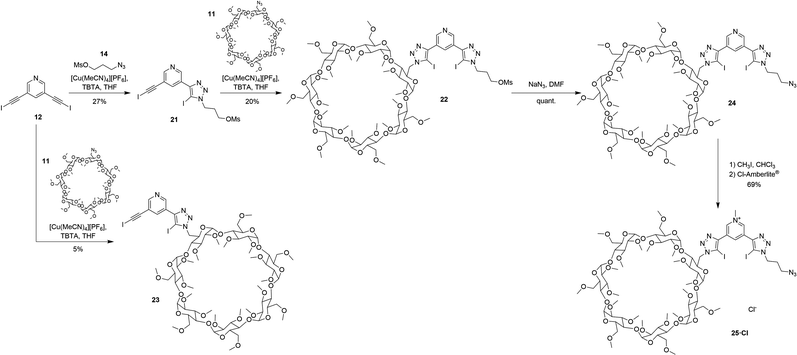
Whilst terphenyl stopper units are suitable for organic and organic–aqueous solvent media, permethylated β-cyclodextrin stopper units are required in order to solubilise a [2]rotaxane analogue in pure water, or solvent mixtures containing a high percentage of water, since permethylation improves the aqueous solubility of β-cyclodextrin.38,39 In a similar manner to rotaxanes 19·PF6 and 20·(PF6)2, β-CD-stoppered rotaxane 27·(OTf)3 was synthesised according to the stepwise procedure shown in Scheme 6 using permethylated β-cyclodextrin functionalised axle component precursors 23 and 25·Cl (Scheme 5). Mono-substitution of 12 with azido-propyl-mesylate 14 was achieved using the same statistical modified CuAAC conditions for the preparation of 13, producing 21 in 27% yield. The CuAAC reaction of 21 with azide functionalised permethylated β-cyclodextrin 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40–42 gave 22 in 20% yield.‡ Conversion of mesylate 22 to the azide 24 was achieved quantitatively using NaN3 in DMF. The pyridinium azide axle precursor 25·Cl was obtained in 69% yield after methylation using CH3I and subsequent anion exchange to the chloride salt using a chloride-loaded Amberlite® column (Scheme 5).
40–42 gave 22 in 20% yield.‡ Conversion of mesylate 22 to the azide 24 was achieved quantitatively using NaN3 in DMF. The pyridinium azide axle precursor 25·Cl was obtained in 69% yield after methylation using CH3I and subsequent anion exchange to the chloride salt using a chloride-loaded Amberlite® column (Scheme 5).
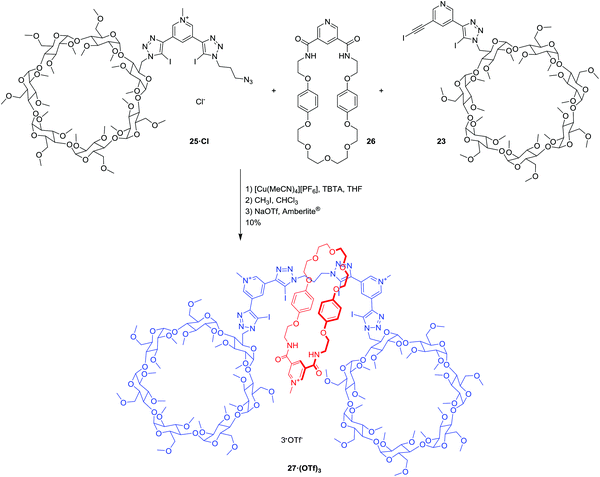
Rotaxane 27·(OTf)3 was prepared using the chloride anion-templated CuAAC click reaction (Scheme 6): mixing equimolar quantities of the two axle precursors 23 and 25·Cl with 10 equivalents of the pyridine bis-amide macrocycle2126 in the presence of Cu(I) and TBTA in THF. The rotaxane was purified using preparative thin layer chromatography (silica, 8% MeOH in CHCl3). Post-rotaxane methylation using CH3I in CHCl3, and subsequent anion exchange to the water-soluble, non-coordinating triflate salt using a triflate-loaded Amberlite® column, afforded the [2]rotaxane 27·(OTf)3 in 10% yield over 3 steps. The tricationic [2]rotaxane 27·(OTf)3 was characterised by 1H, 13C, 19F as well as 2D COSY NMR spectroscopy, high resolution mass spectrometry, and the interlocked nature of the [2]rotaxane was confirmed by 2D ROESY NMR spectroscopic analysis (see ESI†).
Anion binding studies
As a consequence of limited quantities of the mono- and di-cationic [2]rotaxanes 19·PF6 and 20·(PF6)2, respectively, and the tricationic permethylated β-cyclodextrin stopper-axle appended [2]rotaxane 27·(OTf)3, anion binding studies were restricted to chloride and nitrate 1H NMR titration experiments. Whilst anions were titrated as their tetrabutylammonium (TBA) salts for rotaxanes 19·PF6 and 20·(PF6)2, they were added as their sodium salts for the water-soluble rotaxane 27·(OTf)3. In the case of the monocationic [2]rotaxane 19·PF6, a preliminary titration with TBA·NO3 in 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CDCl3
10 CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD
CD3OD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O showed no evidence of binding the oxoanion. Consequently, titrations were repeated in 1
D2O showed no evidence of binding the oxoanion. Consequently, titrations were repeated in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CDCl3
1 CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD, and chemical shifts were monitored for the protons i and 3, incumbent on the anion binding cavity of the rotaxane, as well as protons j and k (Fig. 1a). Analogous titrations for the dicationic [2]rotaxane host 20·(PF6)2 were conducted in 45
CD3OD, and chemical shifts were monitored for the protons i and 3, incumbent on the anion binding cavity of the rotaxane, as well as protons j and k (Fig. 1a). Analogous titrations for the dicationic [2]rotaxane host 20·(PF6)2 were conducted in 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CDCl3
10 CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD
CD3OD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O. Upfield chemical shifts were observed for the pyridinium internal (i and p) and external (j, k and q, r) protons, whilst a downfield shift was observed for the isophthalamide proton 3. Fig. 1b shows the downfield shift for proton j, k of 20·(PF6)2 upon addition of anions. Since the receptor 27·(OTf)3 was only partially soluble in pure water, the anion binding studies were conducted in 9
D2O. Upfield chemical shifts were observed for the pyridinium internal (i and p) and external (j, k and q, r) protons, whilst a downfield shift was observed for the isophthalamide proton 3. Fig. 1b shows the downfield shift for proton j, k of 20·(PF6)2 upon addition of anions. Since the receptor 27·(OTf)3 was only partially soluble in pure water, the anion binding studies were conducted in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 D2O
1 D2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone-d6. Upon addition of chloride, noteworthy downfield shifts were observed for the various pyridinium protons (j, k, l, 2 and 4), whilst proton 3 shifted upfield (Fig. 1c). In contrast, modest upfield shifts were detected for all pyridinium protons upon addition of nitrate to the interlocked host 27·(OTf)3. Job plot approximations and WinEQNMR2
acetone-d6. Upon addition of chloride, noteworthy downfield shifts were observed for the various pyridinium protons (j, k, l, 2 and 4), whilst proton 3 shifted upfield (Fig. 1c). In contrast, modest upfield shifts were detected for all pyridinium protons upon addition of nitrate to the interlocked host 27·(OTf)3. Job plot approximations and WinEQNMR2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 analysis of the titration data for [2]rotaxanes 19·PF6, 20·(PF6)2 and 27·(OTf)3 determined the 1
43 analysis of the titration data for [2]rotaxanes 19·PF6, 20·(PF6)2 and 27·(OTf)3 determined the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric association constants for Cl− and NO3− (Table 1), which were determined to be independent of the proton monitored for a given anion and are compared with the previously mentioned bis-amide HB analogue 28·PF6 (Fig. 2).29
1 stoichiometric association constants for Cl− and NO3− (Table 1), which were determined to be independent of the proton monitored for a given anion and are compared with the previously mentioned bis-amide HB analogue 28·PF6 (Fig. 2).29
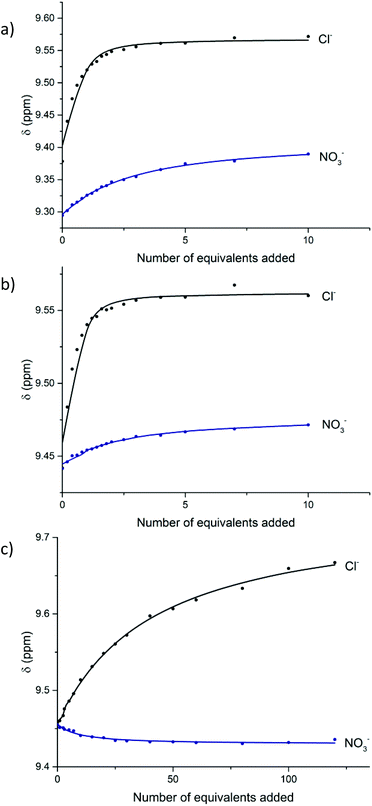 | ||
Fig. 1 Anion binding titrations: (a) 19·PF6 following pyridinium proton j (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CDCl3 1 CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD), (b) 20·(PF6)2 following the external pyridinium protons j, k in the aqueous solvent mixture 45 CD3OD), (b) 20·(PF6)2 following the external pyridinium protons j, k in the aqueous solvent mixture 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CDCl3 10 CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD CD3OD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O, and (c) 27·(OTf)3 following pyridinium proton j. Experimental titration data (circles) with WinEQNMR2 D2O, and (c) 27·(OTf)3 following pyridinium proton j. Experimental titration data (circles) with WinEQNMR2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 fitted binding isotherms indicated as lines (298 K). 43 fitted binding isotherms indicated as lines (298 K). | ||
 | ||
| Fig. 2 Structure of the previously reported HB [2]rotaxane 28·PF6 for nitrate.29 | ||
| Anion |
K
a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (M−1) a (M−1) |
|||
|---|---|---|---|---|
19·PF6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
20·(PF6)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
27·(OTf)3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
28·PF6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
|
a Estimated standard errors are given in parentheses. Association constants calculated using WinEQNMR2 (298 K).43
b 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CDCl3 1 CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD.
c 45 CD3OD.
c 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CDCl3 10 CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD CD3OD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O.
d 9 D2O.
d 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 D2O 1 D2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone-d6. acetone-d6.
|
||||
| Cl− | 7970(1890) | >104 | 112(16) | 490 |
| NO3− | 374(31) | 364(38) | 114(4) | 430 |
The [2]rotaxane 19·PF6 shows strong binding for chloride in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CDCl3
1 CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD. Although binding for nitrate is much weaker than chloride by an order of magnitude, indicating chloride selectivity, the [2]rotaxane host displays notable binding for the oxoanion in this competitive solvent mixture. Moreover, this represents only the second example of nitrate binding by an interlocked halogen-bonding receptor.44
CD3OD. Although binding for nitrate is much weaker than chloride by an order of magnitude, indicating chloride selectivity, the [2]rotaxane host displays notable binding for the oxoanion in this competitive solvent mixture. Moreover, this represents only the second example of nitrate binding by an interlocked halogen-bonding receptor.44
In the relatively more competitive aqueous mixture 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 CDCl3
10 CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD
CD3OD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O, the dicationic XB [2]rotaxane 20·(PF6)2 binds chloride so strongly (Ka > 104 M−1) that it was not possible to determine an accurate Ka value. This is in stark contrast to the previously reported all hydrogen-bonding [2]rotaxane analogue 28·PF6 which exhibited much weaker chloride binding affinity (Ka = 490 M−1). The binding for nitrate with the XB rotaxane 20·(PF6)2 is weaker (Ka = 364 M−1), however, considering the challenge that nitrate recognition represents with its low basicity, the relative strength of association in this aqueous mixture is noteworthy. Interestingly, the halogen-bonding [2]rotaxane 20·(PF6)2 bound nitrate with comparable strength to the previously reported all hydrogen-bonding [2]rotaxane analogue 28·PF6 (Ka = 430 M−1).29
D2O, the dicationic XB [2]rotaxane 20·(PF6)2 binds chloride so strongly (Ka > 104 M−1) that it was not possible to determine an accurate Ka value. This is in stark contrast to the previously reported all hydrogen-bonding [2]rotaxane analogue 28·PF6 which exhibited much weaker chloride binding affinity (Ka = 490 M−1). The binding for nitrate with the XB rotaxane 20·(PF6)2 is weaker (Ka = 364 M−1), however, considering the challenge that nitrate recognition represents with its low basicity, the relative strength of association in this aqueous mixture is noteworthy. Interestingly, the halogen-bonding [2]rotaxane 20·(PF6)2 bound nitrate with comparable strength to the previously reported all hydrogen-bonding [2]rotaxane analogue 28·PF6 (Ka = 430 M−1).29
The tricationic [2]rotaxane 27·(OTf)3 binds both chloride and nitrate in competitive aqueous 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 D2O
1 D2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone-d6 with equal strength: Ka(Cl−) = 112 and Ka(NO3−) = 114 M−1. The incorporation of additional positive charge makes the [2]rotaxane capable of recognising both anions in 90% water, and results in enhanced anion binding ability when compared to the mono- and dicationic [2]rotaxanes. Although the anion binding cavity is evidently complementary towards the trigonal geometry of nitrate, it does not preclude the binding of the spherical halide anion. Presumably, a more rigid axle component joining the two bis-iodotriazole pyridinium groups would preorganise the host to a greater extent and improve the selectivity for nitrate over chloride (and other halide anions). Nonetheless, whilst the receptor shows no selectivity between chloride and nitrate, increasing the percentage of water from 10% to 90% appears to have a much more detrimental influence upon chloride anion binding than the nitrate oxoanion, which reflects the higher hydration enthalpy of chloride (ΔHhyd(Cl−) = −381 kJ mol−1vs. ΔHhyd(NO3−) = −314 kJ mol−1).27,45 Indeed, the relatively strong binding of nitrate in this aqueous mixture presents an important step towards nitrate recognition in pure water.
acetone-d6 with equal strength: Ka(Cl−) = 112 and Ka(NO3−) = 114 M−1. The incorporation of additional positive charge makes the [2]rotaxane capable of recognising both anions in 90% water, and results in enhanced anion binding ability when compared to the mono- and dicationic [2]rotaxanes. Although the anion binding cavity is evidently complementary towards the trigonal geometry of nitrate, it does not preclude the binding of the spherical halide anion. Presumably, a more rigid axle component joining the two bis-iodotriazole pyridinium groups would preorganise the host to a greater extent and improve the selectivity for nitrate over chloride (and other halide anions). Nonetheless, whilst the receptor shows no selectivity between chloride and nitrate, increasing the percentage of water from 10% to 90% appears to have a much more detrimental influence upon chloride anion binding than the nitrate oxoanion, which reflects the higher hydration enthalpy of chloride (ΔHhyd(Cl−) = −381 kJ mol−1vs. ΔHhyd(NO3−) = −314 kJ mol−1).27,45 Indeed, the relatively strong binding of nitrate in this aqueous mixture presents an important step towards nitrate recognition in pure water.
Conclusions
With the objective of demonstrating XB nitrate anion recognition in aqueous media, three novel XB [2]rotaxane anion host systems comprising of XB axle components containing two 3,5-bis-iodotriazole pyridine or pyridinium units and HB isophthalamide or bis-amide pyridinium macrocycles were prepared via a chloride anion-templated mono-stoppering methodology.
1H NMR chloride and nitrate anion binding investigations revealed all three [2]rotaxanes to exhibit an affinity for both anions. Nitrate was bound in solutions containing increasing proportions of water, which correlated with increasing positive charge of the [2]rotaxane host system. While in the case of the monocationic [2]rotaxane 19·PF6, a significantly higher affinity and selectivity for chloride was observed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CDCl3
1 CDCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD, the tricationic permethylated β-cyclodextrin-stoppered [2]rotaxane 27·(OTf)3 displayed equivalent binding affinity for chloride and nitrate in 9
CD3OD, the tricationic permethylated β-cyclodextrin-stoppered [2]rotaxane 27·(OTf)3 displayed equivalent binding affinity for chloride and nitrate in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 D2O
1 D2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone-d6. Importantly, increasing the solvent water percentage from 10% to 90% was shown to have a greater effect upon the binding of chloride than the oxoanion, and correlates with their relative energies of hydration. Whilst the trigonal design of the interlocked XB anion binding site complements the geometry of nitrate, it is still able to accommodate the spherical chloride anion, possibly owing to flexibility in the axle component. These results however represent a significant step towards achieving selective nitrate recognition and illustrate the impressive potential of XB receptors for anion recognition in aqueous media.
acetone-d6. Importantly, increasing the solvent water percentage from 10% to 90% was shown to have a greater effect upon the binding of chloride than the oxoanion, and correlates with their relative energies of hydration. Whilst the trigonal design of the interlocked XB anion binding site complements the geometry of nitrate, it is still able to accommodate the spherical chloride anion, possibly owing to flexibility in the axle component. These results however represent a significant step towards achieving selective nitrate recognition and illustrate the impressive potential of XB receptors for anion recognition in aqueous media.
Acknowledgements
SWR thanks the Clarendon Fund, St John's College, Oxford, and the SCG Innovation Fund for funding.Notes and references
- N. Busschaert, C. Caltagirone, W. Van Rossom and P. A. Gale, Chem. Rev., 2015, 115, 8038–8155 Search PubMed.
- N. H. Evans and P. D. Beer, Angew. Chem., Int. Ed., 2014, 53, 11716–11754 CrossRef CAS PubMed.
- S. Kubik, Chem. Soc. Rev., 2010, 39, 3648–3663 Search PubMed.
- M. J. Langton, C. J. Serpell and P. D. Beer, Angew. Chem., Int. Ed., 2016, 55, 1974–1987 Search PubMed.
- J. L. Sessler, P. A. Gale and W. S. Cho, Anion Receptor Chemistry, Royal Society of Chemistry, 2006 Search PubMed.
- G. R. Desiraju, P. S. Ho, L. Kloo, A. C. Legon, R. Marquardt, P. Metrangolo, P. Politzer, G. Resnati and K. Rissanen, Pure Appl. Chem., 2013, 85, 1711–1713 Search PubMed.
- L. C. Gilday, S. W. Robinson, T. A. Barendt, M. J. Langton, B. R. Mullaney and P. D. Beer, Chem. Rev., 2015, 115, 7118–7195 Search PubMed.
- P. Metrangolo, F. Meyer, T. Pilati, G. Resnati and G. Terraneo, Angew. Chem., Int. Ed., 2008, 47, 6114–6127 Search PubMed.
- P. Metrangolo, T. Pilati, G. Terraneo, S. Biella and G. Resnati, CrystEngComm, 2009, 11, 1187–1196 Search PubMed.
- R. Tepper, B. Schulze, M. Jäger, C. Friebe, D. H. Scharf, H. Görls and U. S. Schubert, J. Org. Chem., 2015, 80, 3139–3150 CrossRef CAS PubMed.
- M. Cametti, K. Raatikainen, P. Metrangolo, T. Pilati, G. Terraneo and G. Resnati, Org. Biomol. Chem., 2012, 10, 1329–1333 Search PubMed.
- M. G. Chudzinski, C. A. McClary and M. S. Taylor, J. Am. Chem. Soc., 2011, 133, 10559–10567 Search PubMed.
- F. Kniep, L. Rout, S. M. Walter, H. K. V. Bensch, S. H. Jungbauer, E. Herdtweck and S. M. Huber, Chem. Commun., 2012, 48, 9299–9301 Search PubMed.
- A. Mele, P. Metrangolo, H. Neukirch, T. Pilati and G. Resnati, J. Am. Chem. Soc., 2005, 127, 14972–14973 Search PubMed.
- A. Caballero, F. Zapata, N. G. White, P. J. Costa, V. Félix and P. D. Beer, Angew. Chem., Int. Ed., 2012, 51, 1876–1880 Search PubMed.
- N. L. Kilah, M. D. Wise, C. J. Serpell, A. L. Thompson, N. G. White, K. E. Christensen and P. D. Beer, J. Am. Chem. Soc., 2010, 132, 11893–11895 Search PubMed.
- B. R. Mullaney, B. E. Partridge and P. D. Beer, Chem. – Eur. J., 2015, 21, 1660–1665 Search PubMed.
- B. R. Mullaney, A. L. Thompson and P. D. Beer, Angew. Chem., Int. Ed., 2014, 53, 11458–11562 CrossRef CAS PubMed.
- C. J. Serpell, N. L. Kilah, P. J. Costa, V. Félix and P. D. Beer, Angew. Chem., Int. Ed., 2010, 49, 5322–5326 Search PubMed.
- F. Zapata, A. Caballero, N. G. White, T. D. W. Claridge, P. J. Costa, V. Félix and P. D. Beer, J. Am. Chem. Soc., 2012, 134, 11533–11541 Search PubMed.
- S. W. Robinson, C. L. Mustoe, N. G. White, A. Brown, A. L. Thompson, P. Kennepohl and P. D. Beer, J. Am. Chem. Soc., 2015, 137, 499–507 Search PubMed.
- A. S. Singh and S.-S. Sun, J. Org. Chem., 2012, 77, 1880–1890 Search PubMed.
- M. M. Watt, L. N. Zakharov, M. M. Haley and D. W. Johnson, Angew. Chem., Int. Ed., 2013, 52, 10275–10280 Search PubMed.
- K. Choi and A. D. Hamilton, J. Am. Chem. Soc., 2001, 123, 2456–2457 Search PubMed.
- A. P. Bisson, V. M. Lynch, M.-K. C. Monahan and E. V. Anslyn, Angew. Chem., Int. Ed. Engl., 1997, 36, 2340–2342 Search PubMed.
- J. Romański and P. Piątek, J. Org. Chem., 2013, 78, 4341–4347 Search PubMed.
- R. Dutta and P. Ghosh, Chem. Commun., 2015, 51, 9070–9084 Search PubMed.
- M. J. Langton and P. D. Beer, Chem. Commun., 2014, 50, 8124–8127 Search PubMed.
- M. J. Langton, L. C. Duckworth and P. D. Beer, Chem. Commun., 2013, 49, 8608–8610 Search PubMed.
- V. Martí-Centelles and P. D. Beer, Chem. – Eur. J., 2015, 21, 9397–9404 Search PubMed.
- L. M. Hancock and P. D. Beer, Chem. – Eur. J., 2009, 15, 42–44 Search PubMed.
- K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467–4470 Search PubMed.
- H. Abe, K. Ohtani, D. Suzuki, Y. Chida, Y. Shimada, S. Matsumoto and M. Inouye, Org. Lett., 2014, 16, 828–831 Search PubMed.
- G. Barker, J. L. McGrath, A. Klapars, D. Stead, G. Zhou, K. R. Campos and P. O'Brien, J. Org. Chem., 2011, 76, 5936–5953 Search PubMed.
- W. S. Brotherton, R. J. Clark and L. Zhu, J. Org. Chem., 2012, 77, 6443–6455 Search PubMed.
- J. E. Hein and V. V. Fokin, Chem. Soc. Rev., 2010, 39, 1302–1315 Search PubMed.
- J. E. Hein, J. C. Tripp, L. B. Krasnova, K. B. Sharpless and V. V. Fokin, Angew. Chem., Int. Ed., 2009, 48, 8018–8021 Search PubMed.
- M. J. Langton, S. W. Robinson, I. Marques, V. Felix and P. D. Beer, Nat. Chem., 2014, 6, 1039–1043 Search PubMed.
- T. Loftsson, P. Jarho, M. Másson and T. Järvinen, Expert Opin. Drug Delivery, 2005, 2, 335–351 Search PubMed.
- J. A. Faiz, N. Spencer and Z. Pikramenou, Org. Biomol. Chem., 2005, 3, 4239–4245 Search PubMed.
- P. J. Skinner, A. Beeby, R. S. Dickins, D. Parker, S. Aime and M. Botta, J. Chem. Soc., Perkin Trans. 2, 2000, 1329–1338 RSC.
- N. Zhong, H. S. Byun and R. Bittman, Tetrahedron Lett., 1998, 39, 2919–2920 Search PubMed.
- M. J. Hynes, J. Chem. Soc., Dalton Trans., 1993, 2, 311 Search PubMed.
- T. A. Barendt, A. Docker, I. Marques, V. Felix and P. D. Beer, Angew. Chem., Int. Ed., 2016, 55, 11069–11076 Search PubMed.
- D. W. Smith, J. Chem. Educ., 1977, 54, 540 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ob02339h |
| ‡ Substitution was ordered in this way due to inclusion of 12 within the cavity of the β-CD-stopper 11, which also explains the reduced yield of the unsymmetrically substituted intermediate 22. However, a nominal quantity of 23 was obtained from the CuAAC click reaction between 12 and 11, which constituted one half of the axle component. |
| This journal is © The Royal Society of Chemistry 2017 |