Open Access Article
Open Access ArticlePhoto-induced interfacial electron transfer of ZnO nanocrystals to control supramolecular assembly in water†
Anna M.
Cieślak
a,
Emma-Rose
Janeček
 b,
Kamil
Sokołowski
a,
Tomasz
Ratajczyk
a,
Michał K.
Leszczyński
a,
Oren A.
Scherman
b,
Kamil
Sokołowski
a,
Tomasz
Ratajczyk
a,
Michał K.
Leszczyński
a,
Oren A.
Scherman
 *b and
Janusz
Lewiński
*b and
Janusz
Lewiński
 *ac
*ac
aInstitute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland
bMelville Laboratory for Polymer Synthesis, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW, UK. E-mail: oas23@cam.ac.uk
cFaculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland. E-mail: lewin@ch.pw.edu.pl
First published on 22nd September 2017
Abstract
Herein, we show how the inherent light-induced redox properties of semiconducting nanocrystals (NCs) can be utilized for the photo-driven reversible modulation of dynamic supramolecular systems formed at their interfaces that, on their own, do not respond to light. This was achieved by the unprecedented combination of photoactive zinc oxide NCs (ZnO NCs) with a host–guest chemistry of cucurbit[8]uril (CB[8]) providing a route to the semiconductor-assisted light modulation of supramolecular assemblies (SALSA), here mediated by the photo-generation of viologen radical cations (MV˙+) at the NC corona and their further dimerization enhanced by CB[8] macrocycles. The reported SALSA strategy was successfully applied for light-controlled reversible assembly processes at NC interfaces enabling light-triggered release of guest molecules from surface confined discrete CB[8] host–guest complexes.
Photo-induced electron transfer (PET) plays a fundamental role in a range of chemical processes, which cover diverse biological systems, transformations of organic and inorganic species, as well as a variety of interfacial electron transfer processes.1–3 PET has found a wide range of applications in emerging technologies across the fields of (photo)catalysis, photovoltaics and electronics.1–3 Intensive development in these areas stems from the incorporation of a variety of nanoparticulate building blocks characterized by unusual physicochemical properties and dynamic processes appearing at their highly extended surface areas. In particular, unique physicochemical properties of semiconductor NCs that can be turned on by light, e.g. interfacial (solid/solution) redox processes (Scheme 1a),2,3 seem to be especially attractive as potential triggers to induce changes in a local chemical environment. While recent efforts to gain fundamental understanding of electron transfer at semiconducting interfaces have been reported,1–3 the implementation of semiconductor-assisted PET processes for the assembly/disassembly of molecular and supramolecular systems is immensely intriguing.
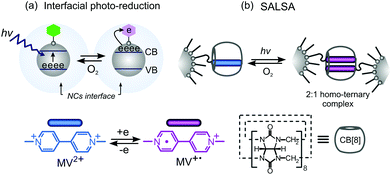 | ||
| Scheme 1 (a) Photo-reduction of organic species at the interfaces of semiconducting NCs;3 (b) the exemplary processes to be attainable via the SALSA strategy. | ||
Within molecular recognition systems, one of the most promising self-assembly motifs in aqueous media relies on interactions between cucurbit[n]urils (CB[n]) and redox active derivatives of 4,4′-bipyridinium dications (viologens, MV2+).4–6 The dimerization of MV˙+ radical cations inside the CB[8] molecular cavity as well as redox controlled stoichiometries of MV2+ based inclusion complexes make these systems a powerful trigger in supramolecular chemistry and dynamic nanosystems, i.e. molecular machines.4,5,7,8 However, while many chemical and electrochemical factors have been used to modulate the affinity of MV2+ derivatives for CB[n] molecular cavities,1,5,7 the use of light as an external stimulus to trigger changes in a completely remote and spatiotemporal manner9–11 is lacking for viologen-based systems.12 Thus, semiconductor-assisted PET processes, which occur at NC interfaces, appear to be particularly attractive for the construction and modulation of dynamic supramolecular interfacial assemblies with CB[8] and MV2+.
To meet this challenge we present a new strategy for semiconductor-assisted light-modulation of supramolecular assemblies, SALSA (Scheme 1b), which is based on the photo-generation of MV˙+ radicals at the interfaces of novel zinc oxide (ZnO) NCs and their further dimerization enhanced by CB[8] macrocycles.7 The PET processes of SALSA were successfully applied to light-controlled assembly processes at the NC interfaces enabling light-triggered release of guest molecules from surface confined discrete CB[8] host–guest complexes. The reversibility of the reported interfacial processes was achieved both through classical chemical factors as well as by light and dioxygen. Additionally, the use of luminescent semiconductor NCs provides a striking visualization method to monitor the release and capture of NC surface-bound guest molecules.
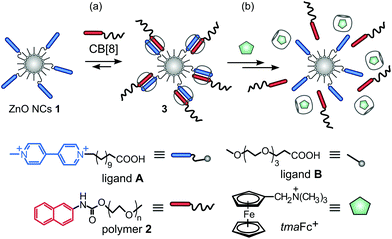
Recently, we have provided novel water soluble ZnO nanocrystalline building blocks stabilized by using a carboxylate oligoethylene glycol shell, which exhibited ultra-long-lived photo-induced charge separation as well as a high propensity for further surface modification and were successfully applied in a hybrid nanosystem for efficient hydrogen evolution.13 We considered these ZnO NCs as ideal constructs for the incorporation of new chemical functionalities at their interfaces, allowing the interplay with CB[n] host–guest chemistry and thereby the supramolecular modulation of nanoparticulate semiconductors. Therefore, in the first step, we employed the reported synthetic approach to achieve an efficient ZnO NC surface grafting with MV2+ recognition sites using 1-methyl-4,4′-bipyridinium-dodecanoic acid bromide iodide, [MV2+-C11-COOH·Br−·I− (A); minor ligand] and 2,5,8,11-tetraoxatetradecan-14-oic acid, [MeO-dPEG(3)-COOH (B); major ligand]. This procedure afforded water-soluble ZnO@MV2+ NCs (ZnO NCs 1) coated with a heterogeneous carboxylate stabilizing shell as depicted in Fig. 1a (for details see the ESI†).
ZnO NCs 1 exhibited a UV absorption band with a maximum (λmax) at 355 nm (Fig. S1†) and yellow luminescence at λmax = 580 nm. The inorganic core of ZnO NCs 1 was roughly spherical and crystalline with a mean diameter of 7.1 ± 1.1 nm as indicated by HRTEM microscopy (6.0 ± 0.2 nm according to PXRD; Fig. S2–S4†). The average hydrodynamic diameter (Dh) was 9 ± 2 nm estimated using dynamic light scattering (DLS; Fig. S5†). In the 1H NMR spectrum of ZnO NCs 1 (D2O) the signals of the MV2+ ligand at δ = 2.08 and δ = 1.45 ppm attributed to α-CH2 and β-CH2 groups, respectively, adjacent to the anchoring carboxylic functionality, are significantly shifted in comparison to the corresponding free ligand A (δ = 2.26 and δ = 1.49 ppm, respectively; Fig. S6†). This suggests that the MV2+ ligands are bound to Zn centers on the ZnO surface.14
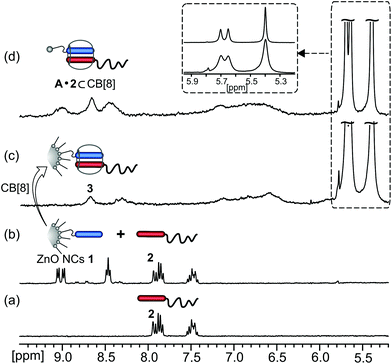
For subsequent experiments on the modification of ZnO NCs 1 by surface confined supramolecular assemblies, it was crucial to demonstrate the accessibility of the interfacial MV2+ recognition sites of ZnO NCs 1 for CB[8] host–guest binding. In order to show that the nanocrystalline surfaces of ZnO NCs 1 can be decorated with discrete host–guest complexes, we employed naphthalene-terminated poly(ethylene glycol) chains [Np-PEG (2)] as representatives of well-established second guests for CB[8] chemistry.6a The addition of CB[8] to an aqueous solution of ZnO NCs 1 and polymer 2 led to the appearance of new bands in the UV-Vis spectrum in the range of 400–600 nm, typical for charge transfer (CT) complexes (Fig. S7†).15 Moreover, the 1H NMR spectrum in D2O of ZnO NCs 1 and polymer 2 in the presence of CB[8] displayed characteristic upfield chemical shifts and the broadening of the viologen and naphthalene moieties’ proton signals, indicating the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hetero-ternary complexes within the ZnO@{MV2+·Np-PEG⊂CB[8]} system (3) as shown in Fig. 2 and S8.†16 Notably, the upfield shift for the CB[8] protons in 3 occurred with signal broadening, in contrast to the analogous conjugates of CB[8] with ligand A and polymer 2, where the signals stemming from CB[8] remained sharp and well resolved (inset in Fig. 2).
1 hetero-ternary complexes within the ZnO@{MV2+·Np-PEG⊂CB[8]} system (3) as shown in Fig. 2 and S8.†16 Notably, the upfield shift for the CB[8] protons in 3 occurred with signal broadening, in contrast to the analogous conjugates of CB[8] with ligand A and polymer 2, where the signals stemming from CB[8] remained sharp and well resolved (inset in Fig. 2).
These observations nicely illustrate that the CB[8] host molecule was included in a larger assembly (thereby reducing its tumbling rate) as a result of the formation of the surface-localized hetero-ternary complexes of 3.16 Notably, UV-Vis and photoluminescence (PL) measurements indicated that the photoluminescence properties of ZnO NCs 1 were preserved upon CB[8]-mediated interfacial functionalization with polymer 2 (Fig. S7 and S10†). Moreover, the resulting interfacial supramolecular system 3 can be disassembled through the use of a competitive guest, such as (ferrocenylmethyl)-trimethylammonium iodide (tmaFc+),17 with a concomitant release of polymer 2 and the regeneration of starting ZnO NCs 1 with photophysical properties intact, as indicated by 1H NMR, UV-Vis and PL spectroscopy (Fig. S11–S13†).
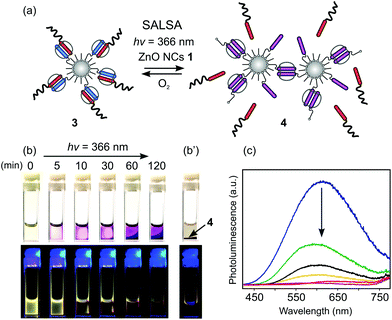
The interplay between redox processes and molecular recognition in CB[8] chemistry has been widely studied and exploited in the design of redox-triggered dynamic supramolecular systems.5,7 With this in mind, we decided to investigate whether the interaction of luminescent ZnO NCs 1 with light will result in the formation of interfacial MV˙+ radical cations,18 providing an alternative way to modulate supramolecular assemblies in water, namely SALSA. Photo-reduction of the MV2+ moieties on ZnO NCs 1 was studied by continuous UV-light (λ = 366 nm) irradiation of an aqueous solution of 1 in the absence of O2. We observed a relatively fast (5 min) appearance of violet color, characteristic of MV˙+ radical cations, that intensified upon further UV-light treatment. UV-Vis spectroscopy measurements revealed new bands at 400 nm and 600 nm indicating the formation of MV˙+ monoradical cations (Fig. S14†).7,19 Subsequently, it was critical to show that photo-generated interfacial MV˙+ species can be utilized for host–guest interactions laying the foundation for SALSA processes. CB[8] macrocycles were added to a water suspension of photo-reduced ZnO NCs 1; this afforded the surface confined 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complexes ZnO@{(MV˙+)2⊂CB[8]} (4) as confirmed by the appearance of new UV-Vis absorption bands at λ = 540 and 960 nm (Fig. S14†).7,20 Strikingly, the photo-generation of MV˙+ monoradical cations is accompanied by the simultaneous switching off of the ZnO luminescence (Fig. S15†).20 Moreover, the formation of ZnO@MV˙+ and subsequent dimerization within the CB[8] cavity can be reversed by the addition of O2 to the solution.
1 inclusion complexes ZnO@{(MV˙+)2⊂CB[8]} (4) as confirmed by the appearance of new UV-Vis absorption bands at λ = 540 and 960 nm (Fig. S14†).7,20 Strikingly, the photo-generation of MV˙+ monoradical cations is accompanied by the simultaneous switching off of the ZnO luminescence (Fig. S15†).20 Moreover, the formation of ZnO@MV˙+ and subsequent dimerization within the CB[8] cavity can be reversed by the addition of O2 to the solution.
We further envisioned that the SALSA approach could be applied for light-triggered switching between hetero- and homo-guest inclusion complexes leading to the controlled uptake and release of target molecules. To demonstrate the versatility of the SALSA approach towards light-induced redox-driven guest-exchange processes, a mixture of ZnO NCs 1 and the supramolecular system 3 with interfacial host–guest hetero ternary complexes (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; based on MV2+ content) in water was prepared (Fig. 3a and S19†). Continuous UV irradiation of the sample for 2 h led to the total switch off of its visible luminescence accompanied by the intensification of the magenta color and the gradual sedimentation of emerging dynamic assemblies 4 (Fig. 3b and c and S16†). The appearance of new UV-Vis absorption bands at λ = 540 and 960 nm suggested the formation of surface confined 2
1; based on MV2+ content) in water was prepared (Fig. 3a and S19†). Continuous UV irradiation of the sample for 2 h led to the total switch off of its visible luminescence accompanied by the intensification of the magenta color and the gradual sedimentation of emerging dynamic assemblies 4 (Fig. 3b and c and S16†). The appearance of new UV-Vis absorption bands at λ = 540 and 960 nm suggested the formation of surface confined 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
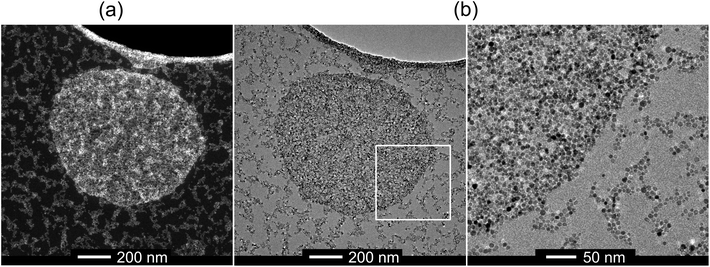
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 homo-inclusion complexes of 4 with a subsequent release of polymer 2 (Fig. S17†).7 After 24 h, a magenta precipitate consisting of ZnO NCs 1 aggregates was collected (Fig. 3b′). In solution, after the redispersion, upon treatment in an ultrasonic bath, the resulting interparticulate assembled system 4 consists of two distinct populations of aggregates with hydrodynamic diameters of 80 ± 10 nm and 800 ± 100 nm as estimated by DLS measurements (Fig. S18†). Additionally, HRTEM measurements show that the larger assemblies of 4 form tight compact clusters, which are surrounded by open, elongated chain-like aggregates (Fig. 4 and S20†).
1 homo-inclusion complexes of 4 with a subsequent release of polymer 2 (Fig. S17†).7 After 24 h, a magenta precipitate consisting of ZnO NCs 1 aggregates was collected (Fig. 3b′). In solution, after the redispersion, upon treatment in an ultrasonic bath, the resulting interparticulate assembled system 4 consists of two distinct populations of aggregates with hydrodynamic diameters of 80 ± 10 nm and 800 ± 100 nm as estimated by DLS measurements (Fig. S18†). Additionally, HRTEM measurements show that the larger assemblies of 4 form tight compact clusters, which are surrounded by open, elongated chain-like aggregates (Fig. 4 and S20†).
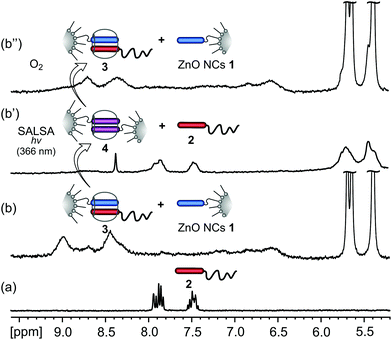
SALSA control over host–guest binding stoichiometry and reversibility of the processes as well as the light-induced release of polymer 2 were further investigated by NMR-tube experiments. In the 1H NMR spectrum of a mixture of ZnO NCs 1 and the supramolecular system 3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; based on MV2+), the signals attributed to the MV2+ moieties of 1, both free and bound within the CB[8] cavity, were significantly broadened (Fig. 5b and S21b). This can be explained by the complex dynamics involving two types of MV2+ species as well as the dynamic substitution of the Np-PEG polymer 2 between different MV2+⊂CB[8] complexes present in system 3. Upon further UV-light (λ = 366 nm; 5 min) irradiation of the sample in the absence of O2 the 1H NMR spectra showed a gradual disappearance of the signals attributed to MV2+ protons indicating the formation of paramagnetic MV˙+ radical cations.21 Extended UV-light treatment (λ = 366 nm; 2 h) of the system resulted in total sample fluorescence quenching and led to the appearance of signals corresponding to the Np moieties of free polymer 2 (Fig. 5b′ and S21c′
1; based on MV2+), the signals attributed to the MV2+ moieties of 1, both free and bound within the CB[8] cavity, were significantly broadened (Fig. 5b and S21b). This can be explained by the complex dynamics involving two types of MV2+ species as well as the dynamic substitution of the Np-PEG polymer 2 between different MV2+⊂CB[8] complexes present in system 3. Upon further UV-light (λ = 366 nm; 5 min) irradiation of the sample in the absence of O2 the 1H NMR spectra showed a gradual disappearance of the signals attributed to MV2+ protons indicating the formation of paramagnetic MV˙+ radical cations.21 Extended UV-light treatment (λ = 366 nm; 2 h) of the system resulted in total sample fluorescence quenching and led to the appearance of signals corresponding to the Np moieties of free polymer 2 (Fig. 5b′ and S21c′![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) †). Additionally, the broadening of the 1H NMR signals attributed to CB[8] protons was observed indicating the formation of 2
†). Additionally, the broadening of the 1H NMR signals attributed to CB[8] protons was observed indicating the formation of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complexes 4 mediated by the dimerization of interfacial MV˙+ radical cation species in the CB[8] molecular cavities. Exposing the resultant system 4 to O2 led to the recovery of its luminescence properties together with the uptake of polymer 2 regenerating the starting surface-localized hetero-ternary complexes of 3, as indicated by 1H NMR and PL (Fig. 5b′′, S16 and S21c′′†).22
1 inclusion complexes 4 mediated by the dimerization of interfacial MV˙+ radical cation species in the CB[8] molecular cavities. Exposing the resultant system 4 to O2 led to the recovery of its luminescence properties together with the uptake of polymer 2 regenerating the starting surface-localized hetero-ternary complexes of 3, as indicated by 1H NMR and PL (Fig. 5b′′, S16 and S21c′′†).22
In conclusion, we have demonstrated, for the first time, that photoactive ZnO NCs can serve as photo-switchable nanoparticulate building blocks for the rich host–guest chemistry of CB[8]. Water-soluble MV2+-functionalized ZnO NCs were successfully modified in a controllable manner through the use of CB[8] as a binding motif resulting in interfacial supramolecular nanosystems, whose assembly can be controlled either by classical chemical factors or through favorable interaction with UV light. In the systems presented, light is a clean, effective and remote stimulus that can readily modulate interfacial host–guest interactions, thus enabling the photo-controlled release of guest molecules as well as the light-induced assembly of more complex supramolecular systems. Through SALSA, the modification and construction of nanomaterial interfaces is driven by the photo-generation of MV˙+ radical cations on the interface of ZnO NC coronas, followed by the formation of {(MV˙+)2⊂CB[8]} binding motifs. SALSA is entirely reversible and the target guest molecules can be further complexed by NCs upon exposure of the system to oxygen. Finally, it was shown that the luminescent semiconductor NCs provided an illuminating possibility to visually monitor the capture and release of guest molecules from surface-bound CB[8] based assemblies. We believe that the reported system paves the way for the development of new, colloidal, multifunctional semiconducting nanostructures for optoelectronics, photovoltaics and catalysis as well as unique photoluminescent tools for biological imaging and sensing in aqueous environments.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
J. Lewiński acknowledges the financial support of the National Science Centre, Poland (OPUS 2014/13/B/ST5/04420). A. M. Cieślak acknowledges the financial support of the National Science Centre, Poland (2015/17/N/ST5/03333) and the Foundation for Polish Science (FNP; START stipend). Prof. J. Waluk and Dr B. Golec are acknowledged for assistance in part of the PL measurements. P. Krupiński is acknowledged for TEM analysis. E.-R. Janeček acknowledges the EPSRC for a PhD stipend and C. S. Y. Tan for the synthesis of polymer 2.Notes and references
- Electron Transfer in Chemistry, ed. V. Balzani, WILEY-VCH Verlag GmbH, 2008, ISBN: 9783527299126 Search PubMed.
- A. Fujishima, X. Zhang and D. Tryk, Surf. Sci. Rep., 2008, 63, 515 CrossRef CAS.
- (a) J. N. Schrauben, R. Hayoun, C. N. Valdez, M. Braten, L. Fridley and J. M. Mayer, Science, 2012, 336, 1298 CrossRef CAS PubMed; (b) H. Zhu, N. Song, W. Rodríguez-Coŕdoba and T. Lian, J. Am. Chem. Soc., 2012, 134, 4250 CrossRef CAS PubMed; (c) J. Moser and M. Grätzel, J. Am. Chem. Soc., 1983, 105, 6547 CrossRef CAS.
- From Non-Covalent Assemblies to Molecular Machines, e. J. P. Sauvage and P. Gaspard, WILEY-VCH Verlag GmbH, 2011, ISBN: 978-3-527-32277-0 Search PubMed.
- The Nature of the Mechanical Bond: From Molecules to Machines, ed. C. J. Bruns and J. F. Stoddart, WILEY-VCH Verlag GmbH, 2016, ISBN: 9781119044000 Search PubMed.
- (a) S. J. Barrow, S. Kasera, M. J. Rowland, J. del Barrio and O. A. Scherman, Chem. Rev., 2015, 115, 12320 CrossRef CAS PubMed; (b) E. Masson, X. Ling, R. Joseph, L. Kyeremeh-Mensah and X. Lu, RSC Adv., 2012, 2, 1213 RSC; (c) Y. Ho Ko, E. Kim, I. Hwang and K. Kim, Chem. Commun., 2007, 1305 RSC; (d) J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, Angew. Chem., Int. Ed., 2005, 44, 4844 CrossRef CAS PubMed; (e) H. Yang, B. Yuan, X. Zhang and O. A. Scherman, Acc. Chem. Res., 2014, 47, 2106 CrossRef CAS PubMed.
- W. S. Jeon, H.-J. Kim, C. Lee and K. Kim, Chem. Commun., 2002, 1828 RSC.
- R. J. Coulston, S. T. Jones, T.-C. Lee, E. A. Appel and O. A. Scherman, Chem. Commun., 2011, 47, 164 RSC.
- (a) G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418 CrossRef CAS PubMed; (b) G. Yu, M. Xue, Z. Zhang, J. Li, C. Han and F. Huang, J. Am. Chem. Soc., 2012, 134, 13248 CrossRef CAS PubMed; (c) Y. Yao, M. Xue, J. Chen, M. Zhang and F. Huang, J. Am. Chem. Soc., 2012, 132, 15712 CrossRef PubMed; (d) G. Yu, X. Zhou, Z. Zhang, C. Han, Z. Mao, C. Gao and F. Huang, J. Am. Chem. Soc., 2012, 134, 19489 CrossRef CAS PubMed.
- (a) J. del Barrio, S. T. J. Ryan, P. G. Jambrina, E. Rosta and O. A. Scherman, J. Am. Chem. Soc., 2016, 138, 5745 CrossRef CAS PubMed; (b) F. Tian, D. Jiao, F. Biedermann and O. A. Scherman, Nat. Commun., 2012, 3, 1207 CrossRef PubMed; (c) J. del Barrio, P. N. Horton, D. Lairez, G. O. Lloyd, C. Toprakcioglu and O. A. Scherman, J. Am. Chem. Soc., 2013, 135, 11760 CrossRef CAS PubMed; (d) C. Hu, Y. Lan, F. Tian, K. R. West and O. A. Scherman, Langmuir, 2014, 30, 10926 CrossRef CAS PubMed.
- (a) R. Klajn, J. F. Stoddart and B. A. Grzybowski, Chem. Soc. Rev., 2010, 39, 2203 RSC; (b) L. Stricker, E.-C. Fritz, M. Peterlechner, N. L. Doltsinis and B. J. Ravoo, J. Am. Chem. Soc., 2016, 138, 4547 CrossRef CAS PubMed; (c) Y. Lan, Y. Wu, A. Karas and O. A. Scherman, Angew. Chem., Int. Ed., 2014, 53, 2166 CrossRef CAS PubMed; (d) H. Zhao, S. Sen, T. Udayabhaskararao, M. Sawczyk, K. Kučanda, D. Manna, P. K. Kundu, J.-W. Lee, P. Král and R. Klajn, Nat. Nanotechnol., 2016, 11, 82 CrossRef CAS PubMed; (e) Q. Zhang, D.-H. Qu, Q.-C. Wang and H. Tian, Angew. Chem., Int. Ed., 2015, 54, 15587 CrossRef; (f) P. K. Kundu, D. Samanta, R. Leizrowice, B. Margulis, H. Zhao, M. Börner, T. Udayabhaskararao, D. Manna and R. Klajn, Nat. Chem., 2015, 7, 646 CrossRef CAS PubMed; (g) R. Klajn, K. J. M. Bishop, M. Fialkowski, M. Paszewski, C. J. Campbell, T. P. Gray and B. A. Grzybowski, Science, 2007, 316, 261 CrossRef CAS PubMed.
- Only very recently, we have noticed that the light-induced aggregation and changes in the catalytic activity of sub-100 nm aggregates of TiO2 NCs were achieved by the application of MV derivatives and CB[8] as the binding motif: Q. Zhang, D.-H. Qu, Q.-C. Wang and H. Tian, Angew. Chem., Int. Ed., 2015, 54, 15587 CrossRef.
- (a) A. M. Cieślak, M. V. Pavliuk, L. D'Amario, M. Abdellah, K. Sokołowski, U. Rybinska, D. L. A. Fernandes, M. K. Leszczyński, F. Mamedov, A. M. El-Zhory, J. Föhlinger, A. Budinská, M. Wolska-Pietkiewicz, L. Hammarström, J. Lewiński and J. Sá, Nano Energy, 2016, 30, 187 CrossRef; (b) M. V. Pavliuk, A. M. Cieślak, M. Abdellah, A. Budinská, S. Pullen, K. Sokolowski, D. Fernandes, J. Szlachetko, E. Bastos, S. Ott, L. Hammarström, T. Edvinsson, J. Lewiński and J. Sá, Sustainable Energy Fuels, 2017, 1, 69 RSC.
- W. Lin, J. Walter, A. Burger, H. Maid, A. Hirsch, W. Peukert and D. Segets, Chem. Mater., 2015, 27, 358 CrossRef CAS.
- (a) H.-J. Kim, J. Heo, W. S. Jeon, E. Lee, J. Kim, S. Sakamoto, K. Yamaguchi and K. Kim, Angew. Chem., Int. Ed., 2001, 40, 1526 CrossRef CAS; (b) J. Kim, I.-S. Jung, S.-Y. Kim, E. Lee, J.-K. Kang, S. Sakamoto, K. Yamaguchi and K. Kim, J. Am. Chem. Soc., 2000, 122, 540 CrossRef CAS.
- F. Biedermann, U. Rauwald, J. M. Zayed and O. A. Scherman, Chem. Sci., 2011, 2, 279 RSC.
- S. Liu, C. Ruspic, P. Mukhopadhyay, S. Chakrabarti, P. Y. Zavalij and L. Isaacs, J. Am. Chem. Soc., 2005, 127, 15959 CrossRef CAS PubMed.
- (a) A. J. Morris-Cohen, M. T. Frederick, L. C. Cass and E. A. Weiss, J. Am. Chem. Soc., 2011, 133, 10146 CrossRef CAS PubMed; (b) L. Cusack, X. Marguerettaz, S. N. Rao, J. Wenger and D. Fitzmaurice, Chem. Mater., 1997, 9, 1765 CrossRef CAS.
- This observation is in agreement with a control experiment involving the MV2+-functionalized ligand A and sodium dithionite Na2S2O4 as a reducing agent (Fig. S14†).
- (a) A. M. Schimpf, C. E. Gunthardt, J. D. Rinehart, J. M. Mayer and D. R. Gamelin, J. Am. Chem. Soc., 2013, 135, 16569 CrossRef CAS PubMed; (b) A. W. Cohn, N. Janßen, J. M. Mayer and D. R. Gamelin, J. Phys. Chem. C, 2012, 116, 20633 CrossRef CAS.
- W. S. Jeon, E. Kim, Y. Ho Ko, I. Hwang, J. W. Lee, S.-Y. Kim, H.-J. Kim and K. Kim, Angew. Chem., Int. Ed., 2005, 44, 87 CrossRef CAS PubMed.
- We note that the interfacial photo-generation of MV˙+ radical species followed by SALSA processes are completely reversible towards O2 treatment at every stage of the described in situ experiments. However, once 4 precipitates from solution, its further re-dispersion is difficult, which is more likely an effect of other forces emerging in the system during aggregate formation, i.e. cooperative weak interactions between stabilizing ligand chains of the adjacent NCs.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, detailed synthetic and experimental procedures and characterization. See DOI: 10.1039/c7nr03095a |
| This journal is © The Royal Society of Chemistry 2017 |