Efficient nickel(ii) naringenin-oxime complex catalyzed Mizoroki–Heck cross-coupling reaction in the presence of hydrazine hydrate†
Abstract
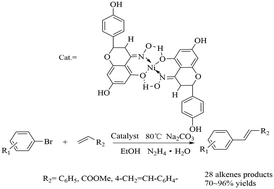
A novel nickel(II) naringenin-oxime complex was designed, synthesized and characterized. Therein, the nickel(II) naringenin oxime complex as an efficient catalyst was used in Mizoroki–Heck coupling reactions of aryl halides containing electron-rich and electron-deficient substituents with styrene, methyl acrylate and divinylbenzene (DVB), respectively. The reaction proceeded efficiently under alkaline conditions in the presence of 0.30 mol% of the Ni(II) naringenin oxime complexand N2H4·H2O as the reductant in EtOH at 80 °C, and 32 alkene products were afforded in moderate to excellent yields, containing four new olefins. The new catalytic system not only provided an inexpensive and efficient process with greener conditions, but also broadened the reaction scope.


 Please wait while we load your content...
Please wait while we load your content...