N-Methyl-N,N,N-trioctylammonium chloride as a novel and green corrosion inhibitor for mild steel in an acid chloride medium: electrochemical, DFT and MD studies†
Abstract
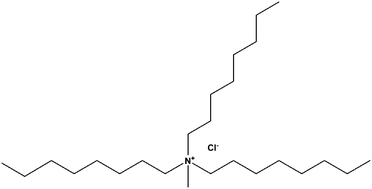
In the present work, N-methyl-N,N,N-trioctylammonium chloride (Aliquat 336) has been evaluated as a green and novel corrosion inhibitor for mild steel in a 1 M HCl solution using electrochemical impedance spectroscopy (EIS), potentiodynamic polarization (PDP) and chemical, spectroscopic (FTIR and UV-visible) methods. The results show that Aliquat 336 is an effective corrosion inhibitor and showed the maximum inhibition efficiency of 94.6% at a very low concentration of 4.95 μM. The results of the EIS study showed that Aliquat 336 inhibits corrosion by an adsorption mechanism. The PDP results revealed that Aliquat 336 is a mixed type inhibitor, which predominantly acts as a cathodic inhibitor. The formation of an inhibitor film on the metal surface was corroborated by atomic force microscopy (AFM). The adsorption of Aliquat 336 on a mild steel surface obeys the Langmuir adsorption isotherm. Quantum chemical calculations (DFT based) and molecular dynamics (MD) simulation studies further corroborated the adsorption and inhibition action of Aliquat 336 on the steel surface.


 Please wait while we load your content...
Please wait while we load your content...