 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fullerene C60 dianion salt, (Me4N+)2(C602−)·(TPC)2·2C6H4Cl2, where TPC is triptycene, obtained by a multicomponent approach†
Dmitri V.
Konarev
 *a,
Sergey I.
Troyanov
*a,
Sergey I.
Troyanov
 b,
Akihiro
Otsuka
cd,
Hideki
Yamochi
cd,
Gunzi
Saito
ef and
Rimma N.
Lyubovskaya
a
b,
Akihiro
Otsuka
cd,
Hideki
Yamochi
cd,
Gunzi
Saito
ef and
Rimma N.
Lyubovskaya
a
aInstitute of Problems of Chemical Physics RAS, Chernogolovka, 142432, Russia. E-mail: konarev3@yandex.ru; Fax: +7-496-522-18-52
bMoscow State University, Leninskie Gory, 119991 Moscow, Russia
cResearch Center for Low Temperature and Materials Sciences, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan
dDivision of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan
eFaculty of Agriculture, Meijo University, 1-501 Shiogamaguchi, Tempaku-ku, Nagoya 468-8502, Japan
fToyota Physical and Chemical Research Institute, 41-1, Yokomichi, Nagakute, Aichi 480-1192, Japan
First published on 11th May 2017
Abstract
A multicomponent approach provided a new salt (Me4N+)2(C602−)·(TPC)2·2C6H4Cl2 (1) containing the discrete C602− dianions, where TPC is triptycene. The crystal structure consists of alternating layers of closely packed zigzag fullerene chains solvated with C6H4Cl2 molecules and the TPC network with Me4N+ cations. The hexagonal vacancies in the TPC network accommodate the cations and simultaneously act as a template for the formation of zigzag fullerene chains. Optical spectra support the formation of C602− dianions. According to the electron paramagnetic resonance technique (EPR) the C602− dianions have a diamagnetic singlet ground state below 140 K. The increased intensity of the EPR signal above this temperature reflects the growing thermal population of the excited triplet state. This signal has g = 2.0003 and a linewidth of 2.53 mT at 302 K and the estimated singlet–triplet energy gap is 674 ± 10 K (468 ± 7 cm−1).
Fullerene compounds possess promising conducting and magnetic properties.1–4 For example, compounds with monoreduced fullerenes obtained by alkali metal doping,1 layered salts with hexagonal C60˙− packing,2 and fullerene complexes with partial charge transfer3 show metallic conductivity whereas fullerene salt with tetrakis(dimethylamino)ethylene manifests ferromagnetic ordering of spins below 16 K.4 It is known5 that the C60˙− radical anions have a strong tendency to form diamagnetic singly-bonded fullerene (C60−)2 dimers. Therefore, an important point in the design of such compounds is to organize close packing of fullerene anions in a crystal but steric hindrances should be introduced to prevent the dimerization of fullerenes.
The effectiveness of the multicomponent approach is proved to form the abovementioned hexagonal C60˙− packing. In this way, one of the components is a structure-forming molecule of large size, which defines the crystal structure of the compound and prevents fullerene dimerization. Another component is a cation of small size, which defines the charged state of fullerene. Up to now several compounds of such type have been obtained,2,6 for example, (MDABCO+)(C60˙−)·TPC,2 where MDABCO+ is the N-methyldiazabicyclooctanium cation and TPC is a structure-forming triptycene molecule. TPC molecules form a hexagonal network which accommodates the MDABCO+ cations and becomes a template for the formation of closely packed hexagonal fullerene layers. At the same time fullerenes are separated in the layers by TPC molecules and cannot dimerize even at short distances between them. As a result, this compound shows metallic conductivity in the fullerene layers preserved from room temperature down to liquid helium temperatures. This is a rare example of a fullerene compound with quasi-two-dimensional metallic conductivity.2 Variation of cations allows the interfullerene distances in the layers to change, and (MQ+)(C60˙−)·TPC with larger N-methylquinucledinium cations transfers to the Mott insulating state.6c Recently this approach was extended to the synthesis of layered salts7 with metal phthalocyanines allowing one to obtain compounds with strong magnetic coupling of spins in (Me4P+)[MIVO(Pc˙3−)]˙−(TPC)0.5·C6H4Cl2 (M = Ti and V, Pc is phthalocyanine).7b
Despite the fact that different fullerene salts with the C602− dianions were obtained,8 the multi-component salts with the C602− dianions are still unknown. In this work we for the first time extend the multicomponent approach to the synthesis of dianion fullerene salt to obtain crystalline (Me4N+)2(C602−)·(TPC)2·2C6H4Cl2 (1) with closely packed zigzag fullerene chains. The optical and magnetic properties of 1 and the effectiveness of structural design of the multicomponent dianion salt are discussed.
The salt was synthesized using a strong reductant sodium fluorenone ketyl9 which can generate the C602− dianions in solution in the presence of an excess of the Me4N+ cations.8l Slow mixing of the obtained solution with n-hexane without TPC did not yield the crystals of the two-component salt (Me4N+)2(C602−). The synthesis in the presence of an excess of TPC resulted in the crystallization of thin plates in high yield whose composition was determined by X-ray diffraction on a single crystal (using synchrotron radiation) to be (Me4N+)2(C602−)·(TPC)2·2C6H4Cl2 (1).
The charged state of fullerene can be unambiguously determined from the spectra in the IR- and visible-NIR ranges10 measured in KBr pellets prepared under anaerobic conditions. A 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cation to fullerene ratio indicates a formal 2− charge on the fullerene molecules, which is supported by the IR spectrum of 1. The absorption bands of C60 are manifested in the spectrum of 1 at 516, 572, 1184 and 1371 cm−1 assignable to the F1u(1–4) modes, respectively. The band of the F1u(4) C60 mode is most sensitive to charge transfer to the fullerene molecule and is shifted from 1429 cm−1 (neutral state) to 1388–1396 cm−1 in the spectra of the salts with the C60˙− radical anions and to 1370–1374 cm−1 in the spectra of the salts with the C602− dianions.10 The position of this band at 1371 cm−1 in the spectrum of 1 clearly indicates the formation of C602−. Fullerene C60 anions in each charged state show characteristic spectra in the near-IR range.10d,e The presence of absorption bands at 845 and 956 nm (Fig. 1) confirms the dianionic state of C60. The spectrum of 1 also contains a broad and relatively weak band with a maximum at 1220 nm (shown by an arrow in Fig. 1). Most probably this band can be attributed to the interfullerene charge transfer process in closely packed zigzag fullerene chains (see the crystal structure section). Generally, such charge transfer bands are manifested in the spectra of the salts containing closely packed fullerene anions.6c
1 cation to fullerene ratio indicates a formal 2− charge on the fullerene molecules, which is supported by the IR spectrum of 1. The absorption bands of C60 are manifested in the spectrum of 1 at 516, 572, 1184 and 1371 cm−1 assignable to the F1u(1–4) modes, respectively. The band of the F1u(4) C60 mode is most sensitive to charge transfer to the fullerene molecule and is shifted from 1429 cm−1 (neutral state) to 1388–1396 cm−1 in the spectra of the salts with the C60˙− radical anions and to 1370–1374 cm−1 in the spectra of the salts with the C602− dianions.10 The position of this band at 1371 cm−1 in the spectrum of 1 clearly indicates the formation of C602−. Fullerene C60 anions in each charged state show characteristic spectra in the near-IR range.10d,e The presence of absorption bands at 845 and 956 nm (Fig. 1) confirms the dianionic state of C60. The spectrum of 1 also contains a broad and relatively weak band with a maximum at 1220 nm (shown by an arrow in Fig. 1). Most probably this band can be attributed to the interfullerene charge transfer process in closely packed zigzag fullerene chains (see the crystal structure section). Generally, such charge transfer bands are manifested in the spectra of the salts containing closely packed fullerene anions.6c
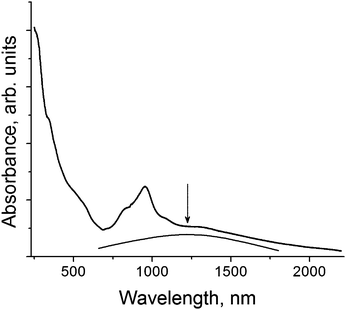 | ||
| Fig. 1 Spectrum of salt 1 in the UV-visible-NIR ranges in KBr pellets prepared under anaerobic conditions. | ||
The crystal structure of 1 was determined at 100 K.‡ The independent components are shown in Fig. 2. The C602− dianion is located on a twofold axis and is statistically disordered between two orientations. Salt 1 has a layered structure which is characteristic of all the compounds obtained by the multi-component approach with TPC2,6c,7 (Fig. 3). The organic layer contains a hexagonal TPC network which accommodates one small Me4N+ cation in each vacancy (Fig. 3b). The network is not ideally hexagonal as in the case of (MDABCO+)(C60˙−)·TPC with the MDABCO+ cations2 but is slightly distorted. The triangles formed by three nitrogen atoms of Me4N+ in this network have the sides of 9.01, 9.87 and 10.30 Å. Fullerene C602− dianions are partially penetrated into the hexagonal TPC network which becomes a template to build fullerene layers. Fullerene layers contain zigzag fullerene chains arranged along the c axis and alternate with the o-C6H4Cl2 molecules along the b axis (Fig. 3a). These chains can be considered as fragments of closely packed hexagonal fullerene layers since the zigzag angle is close to 120° and the center-to-center interfullerene distances are only 10.015 and 10.031 Å (very close to those in the closely packed hexagonal fullerene layers of (MDABCO+)(C60˙−)·TPC – 10.07 Å at 300 K).2 However, hexagonal fullerene layers are not formed in 1 since there are two hexagonal vacancies occupied by Me4N+ per one fullerene unit due to the dianion state of fullerenes. As a result, one of two fullerenes in the hexagonal layer is substituted by two neutral C6H4Cl2 molecules to retain the electroneutrality of the compound. In 1, the zigzag fullerene chains with an angle of 120° are the resultant packing patterns of fullerene dianions.
The magnetic properties of the salt were studied by the EPR technique for a polycrystalline sample of 1 sealed in the quartz tube. Only a weak narrow EPR signal is observed below 140 K with a nearly temperature independent g-factor of 1.9999 and a linewidth (ΔH) of 0.4–0.5 mT. The integral intensity of this signal corresponds to the contribution of less than 2% of spins from the total amount of C60. This indicates the diamagnetic singlet ground state for the C602− dianions in 1. Above 140 K a new signal grows in intensity and it is strongly broadened with the temperature increase (Fig. 4b and c). The intensity increases more than 3 times and the signal has g = 2.0003 and ΔH = 2.53 mT at 302 K (Fig. 4a). Such behaviour can be attributed to the thermal population of the excited triplet state of the C602− dianions. The triplet features of the signal are not manifested at high temperature (T > 140 K) most probably due to averaging effects. The plot of natural logarithm of integral intensity multiplied by temperature vs. reverse temperature is linear in the 300–180 K range allowing one to estimate the singlet–triplet energy gap in the C602− dianions to be 674 ± 10 K (468 ± 7 cm−1). Previously several salts containing the C602− dianions manifested the diamagnetic singlet ground state and thermal population of the excited triplet state with the singlet–triplet energy gaps in the 701–1045 K or 487–730 cm−1 ranges.8j,l It is seen that the gap obtained for 1 is the smallest gap among all studied C602− salts. Nevertheless, the population of the excited triplet state in 1 is less than 10% of spins from the total amount of C60. This is too small even at 302 K to manifest the conductivity or promising magnetic properties.
It is evident that the multi-component approach can be used in the design of dianion fullerene salts. As in the case of previously studied multi-component C60˙− salts2,6 TPC molecules form a hexagonal network with vacancies accommodating small Me4N+ cations. This network becomes a template to build fullerene layers which contains closely packed zigzag fullerene chains and solvent o-C6H4Cl2 molecules. Potentially the TPC network allows the formation of hexagonal fullerene layers but in the case of the C602− dianions only fragments of these layers are formed since half of fullerene positions in these layers are substituted by neutral solvent molecules to preserve the electroneutrality of the compound. It is shown that the C602− dianions have the singlet ground state in 1 with the excited triplet state thermally populated above 140 K. The singlet–triplet energy gap was estimated to be 674 ± 10 K. To date this is the smallest gap among studied C602− salts.
This work was supported by FASO Russia, state task #0089-2014-0036 and JSPS KAKENHI Grant Numbers JP23225005 and JP26288035.
Notes and references
- (a) P. W. Stephens, G. Bortel, G. Faigel, M. Tegze, A. Jánossy, S. Pekker, G. Oszlanyi and L. Forró, Nature, 1994, 370, 636 CrossRef CAS; (b) F. Bommeli, L. Degiorgi, D. Wachter, Ö. Legeza, A. Jánossy, G. Oszlanyi, O. Chauvet and L. Forro, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 51, 14794 CrossRef CAS; (c) M. J. Rosseinsky, J. Mater. Chem., 1995, 5, 1497 RSC.
- D. V. Konarev, S. S. Khasanov, A. Otsuka, M. Maesato, G. Saito and R. N. Lyubovskaya, Angew. Chem., Int. Ed., 2010, 49, 4829 CrossRef CAS PubMed.
- D. V. Konarev, S. S. Khasanov, M. Ishikawa, E. I. Yudanova, A. F. Shevchun, M. S. Mikhailov, P. A. Stuzhin, A. Otsuka, H. Yamochi, G. Saito and R. N. Lyubovskaya, ChemistrySelect, 2016, 1, 323 CrossRef CAS.
- P. W. Stephens, D. Cox, J. W. Lauher, L. Mihaly, J. B. Wiley, P.-M. Allemand, A. Hirsch K. Holczer, Q. Li, J. D. Thompson and F. Wudl, Nature, 1992, 355, 331 CrossRef CAS.
- (a) D. V. Konarev, S. S. Khasanov, G. Saito, A. Otsuka, Y. Yoshida and R. N. Lyubovskaya, J. Am. Chem. Soc., 2003, 125, 10074 CrossRef CAS PubMed; (b) D. V. Konarev, S. S. Khasanov and R. N. Lyubovskaya, Russ. Chem. Bull., 2007, 56, 371 CrossRef CAS; (c) G. A. Domrachev, Y. A. Shevelev, V. K. Cherkasov, G. V. Markin, G. K. Fukin, S. Ya. Khorshev, B. S. Kaverin and V. L. Karnatchevich, Russ. Chem. Bull., 2004, 53, 2056 CrossRef CAS; (d) N. V. Kozhemyakina, K. Y. Amsharov, J. Nuss and M. Jansen, Chem. – Eur. J., 2011, 17, 1798 CrossRef CAS PubMed.
- (a) D. V. Konarev, A. Y. Kovalevsky, S. S. Khasanov, G. Saito, A. Otsuka and R. N. Lyubovskaya, Eur. J. Inorg. Chem., 2005, 4822 CrossRef CAS; (b) D. V. Konarev, S. S. Khasanov, G. Saito, A. Otsuka and R. N. Lyubovskaya, J. Mater. Chem., 2007, 17, 4171 RSC; (c) D. V. Konarev, S. S. Khasanov, A. Otsuka, M. Maesato, M. Uruichi, K. Yakushi, A. Shevchun, H. Yamochi, G. Saito and R. N. Lyubovskaya, Chem. – Eur. J., 2014, 20, 7268 CrossRef CAS PubMed.
- (a) D. V. Konarev, S. S. Khasanov, M. Ishikawa, A. Otsuka, H. Yamochi, G. Saito and R. N. Lyubovskaya, Inorg. Chem., 2013, 52, 3851 CrossRef CAS PubMed; (b) D. V. Konarev, S. S. Khasanov, A. V. Kuzmin, Y. Nakano, M. Ishikawa, A. Otsuka, H. Yamochi, G. Saito and R. N. Lyubovskaya, Cryst. Growth Des., 2017, 17, 753 CrossRef CAS.
- (a) P. Paul, Z. Xie, R. Bau, P. D. W. Boyd and C. A. Reed, J. Am. Chem. Soc., 1994, 116, 4145 CrossRef CAS; (b) J. Hong, M. P. Shores and C. M. Elliott, Inorg. Chem., 2010, 49, 11378 CrossRef CAS PubMed; (c) K. Himmel and M. Jansen, Eur. J. Inorg. Chem., 1998, 1183 CrossRef CAS; (d) K. Himmel and M. Jansen, Chem. Commun., 1998, 1205 RSC; (e) K. Himmel and M. Jansen, Z. Anorg. Allg. Chem., 1998, 624, 1 CrossRef CAS; (f) K. Himmel and M. Jansen, Inorg. Chem., 1998, 37, 3437 CrossRef CAS; (g) H. Brumm and M. Jansen, Z. Anorg. Allg. Chem., 2001, 627, 1433 CrossRef CAS; (h) T. F. Fässler, A. Spiekermann, M. E. Spahr and R. Nesper, Angew. Chem., Int. Ed., 1997, 36, 486 CrossRef; (i) S. D. Hoffmann and T. F. Fässler, Z. Naturforsch., B: Chem. Sci., 2004, 59, 1579 CAS; (j) D. V. Konarev, S. S. Khasanov, I. I. Vorontsov, G. Saito, Y. M. Antipin and R. N. Lyubovskaya, Inorg. Chem., 2003, 42, 3706 CrossRef CAS PubMed; (k) D. V. Konarev, S. S. Khasanov, G. Saito and R. N. Lyubovskaya, J. Porphyrins Phthalocyanines, 2008, 12, 1146 CrossRef CAS; (l) D. V. Konarev, A. V. Kuzmin, S. V. Simonov, S. S. Khasanov, E. I. Yudanova, G. Saito and R. N. Lyubovskaya, Phys. Chem. Chem. Phys., 2013, 15, 9136 RSC.
- R. O. Loutfyc, K. Hsiaob, S. Ong and B. Keoshkeria, Can. J. Chem., 1984, 62, 1877 CrossRef.
- (a) M. S. Dresselhaus, G. Dresselhaus and P. C. Eklund, Science of Fullerenes and Carbon Nanotubes, Academic Press, San Diego, 1996 Search PubMed; (b) T. Picher, R. Winkler and H. Kuzmany, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 15879 CrossRef; (c) V. N. Semkin, N. G. Spitsina, S. Krol and A. Graja, Chem. Phys. Lett., 1996, 256, 616 CrossRef CAS; (d) C. A. Reed and R. D. Bolskar, Chem. Rev., 2000, 100, 1075 CrossRef CAS PubMed; (e) D. V. Konarev and R. N. Lyubovskaya, Russ. Chem. Rev., 2012, 81, 336 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: IR spectra of starting compounds and salt 1. CCDC 1539600. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7nj01096f |
‡ Crystal data for 1: C120H60Cl4N2, F.W. 1671.50, brown plate, 0.4 × 0.4 × 0.01 mm3; 100(2) K: orthorhombic, space group Pbcn, a = 24.182(2), b = 17.718(1), c = 17.929(1) Å, V = 7681.8(9) Å3, Z = 4, dcalcd = 1.445 g cm−3, μ = 0.396 mm−1, F(000) = 3448, 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 058 reflections collected, 8707 independent; R1 = 0.129 for 4947 observed data [>2σ(F)] with 702 restraints and 707 parameters; wR2 = 0.360 (all data); G.o.F. = 1.013. CCDC 1539600. 058 reflections collected, 8707 independent; R1 = 0.129 for 4947 observed data [>2σ(F)] with 702 restraints and 707 parameters; wR2 = 0.360 (all data); G.o.F. = 1.013. CCDC 1539600. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |



