Contrasting effects of heterocycle substitution and branched tails in the arms of star-shaped molecules†
Abstract
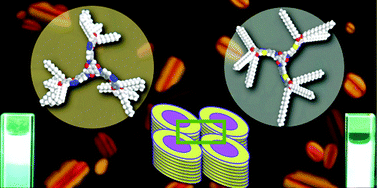
Herein, star-shaped tris(N-salicylideneaniline)s (TSANs) containing 1,3,4-oxadiazole based and 1,3,4-thiadiazole based arms are synthesized and characterized. The introduction of branched tails at their peripheries has different effects on these tris(N-salicylideneaniline)s, which are dependent on the type of heterocycle. The TSANs bearing 1,3,4-oxadiazole arms with branched tails exhibit a room temperature columnar rectangular phase in comparison to the high temperature columnar hexagonal phase exhibited by their hexadecyloxy chain analogues. In the case of the thiadiazole based TSANs, the compound with hexadecyloxy chains exhibits a columnar rectangular phase over a wide temperature range including room temperature, whereas its branched chain analogue is a liquid. Thus, in the case of star-shaped molecules, the type of peripheral tails not only affects the transition temperature, but also affects the type of self-assembly, which is in contrast to conventional discotic liquid crystals. The introduction of substituted 1,3,4-thiadiazole rings helps in the reduction of their melting points and enhances their mesophase width. The presence of bulky branched tails at their peripheries enhances the intermolecular interactions between the cores of the 1,3,4-oxadiazole based TSANs, which leads to the stabilization of the columnar rectangular phase. The 1,3,4-thiadiazole based TSANs exhibit a columnar rectangular phase even with straight peripheral chains because of the attractive intermolecular interactions of the thiadiazole ring.


 Please wait while we load your content...
Please wait while we load your content...