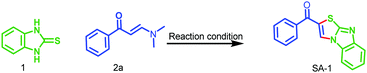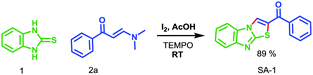Iodine-mediated C–N and C–S bond formation: regioselective synthesis of benzo[4,5]imidazo[2,1-b]thiazoles†
Sethurajan
Ambethkar
a,
Muthalu
Vellimalai
a,
Vediappen
Padmini
*a and
Nattamai
Bhuvanesh
b
aDepartment of Organic Chemistry, School of Chemistry, Madurai Kamaraj University, Madurai 625021, Tamilnadu, India. E-mail: padmini.chem@mku.org; padimini_tamilenthi@yahoo.co.in
bX-ray Diffraction Laboratory, Department of Chemistry, Texas A & M University, College Station, TX 77842, USA
First published on 3rd November 2016
Abstract
A new strategy involving iodine-mediated reactions for the construction of C–N and C–S bonds has been reported. This method is base and metal free and features inexpensive catalysts, with a simple procedure and a short reaction time.
Introduction
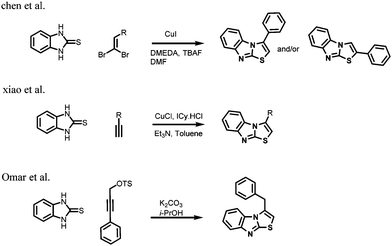
Oxidative C–H bond activation/functionalization has emerged as an effective tool for the construction of carbon–carbon and carbon–heteroatom bond formations by using transition metals as catalysts.1,2 Metal free organic synthesis for the construction of C–C and C–X bonds are also popular.3 Iodine seems to be an excellent reagent owing to its characteristics such as ready availability, low cost and non-metallic nature. Moreover, molecular iodine has additional advantages as it has the lowest dissociation energy, no radioactivity and a moderate redox potential.4 The use of iodine in oxidative coupling reactions has been extensively explored. Specifically, molecular iodine has been extensively used for C–S5 coupling reactions involving sulfonylating reagents such as DMSO, R-SO2NHNH2, R′SO2Na and R2S2. Furthermore, Li et al.6 reported iodine-mediated intramolecular cyclization of enamines via iodide intermediates.Benzimidazo[2,1-b]thiazoles are privileged structures found in a wide range of natural products and bioactive molecules. This class of compounds exhibits diverse biological activities such as antibacterial,7 antitumor,8 antidiabetic,9 anti-inflammatory,10 antitubercular,11 anti-cardiovascular,12 anti-neurodegenerative13 and immunosuppressive activities.14 A number of synthetic methods have been developed for the synthesis of benzimidazo[2,1-b]thiazoles including copper(I) catalyzed arylation,15 Pd–Cu catalyzed heterocyclization16 and carbene rearrangement of hypervalent bromanes.17 In 2010 Chen et al.18 developed a powerful copper(I) catalysed aminothiolation reaction of 1,1-dihaloalkene, and later in 2012 Xiao et al.19 established a new method for the synthesis of N-fused heterocycles via C–S coupling and 5-endo-dig cyclization reactions (Scheme 1). Recently, Gabriele et al.20 reported palladium-catalyzed carbonylative multicomponent synthesis of functionalized benzimidazothiazoles (Fig. 1).
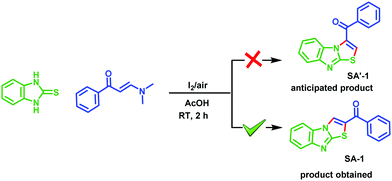
However, there is no report available for the synthesis of benzimidazothiazoles starting from enamines. Enaminones are versatile synthons for organic synthesis and their chemistry has received much attention in recent years. They are ambient in nature and, due to the nucleophilicity of enamine and the electrophilicity of enone, they find applications as building blocks for the construction of a large number of organic compounds.21 Thus enaminones have been chosen as the starting material for the present investigation to obtain benzimidazothiazoles. The enamino ketones, (E)-1-(4-aryl)-3-(dimethylamino)-prop-2-en-1-ones, required for the present work, were prepared following previously reported methods.22 To the best of our knowledge, this is the first report for the simultaneous construction of C–N and C–S bonds in a single operation using molecular iodine (Scheme 2).
Results and discussion
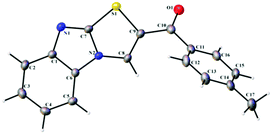
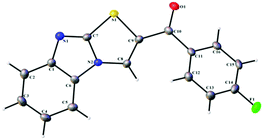
In continuation of our ongoing research in the development of new routes to access heterocyclic systems,23 we started our investigation by utilizing 2-mercaptobenzimidazole 1 (1.0 equiv.) and (E)-1-(4-aryl)-3-(dimethylamino)-prop-2-en-1-one 2a (1.0 equiv.) as model substrates for the synthesis of benzo[4,5]imidazo[2,1-b]thiazole (SA-1) under various reaction conditions as depicted in Table 1. When the reaction was carried out in 5 mL of acetic acid at room temperature for 6 h, no product was formed (Table 1, entry 1). Further optimization revealed that the use of CuI (1.0 equiv.) as a promoter in acetic acid at room temperature under open air for 12 h afforded the product in 62% yield (Table 1, entry 2). Here we expected SA′-1 as a product; because sulphur is a stronger nucleophile compared to nitrogen in 2-mercaptobenzimidazole, it can undergo thione–thiol tautomerization. There could be a nucleophilic attack of (E)-3-(dimethylamino)-1-phenylprop-2-en-1-one with 1H-benzo[d]imidazole-2-thiol, which could lead to the elimination of N,N-dimethylamine, and then intramolecular cyclization could afford the expected product SA′-1. Unfortunately the expected product SA′-1 was not formed. The unexpected product, SA-1, was formed via the nucleophilic substitution reaction and intramolecular oxidative cyclization, followed by elimination of Me2NH as a by-product, as shown in Scheme 2. The structure of SA-1 was undoubtedly confirmed by the spectral and single crystal X-ray data (Fig. 2 and 3). Next we optimized equimolar amounts of various copper reagents such as CuI, CuCl, CuBr, CuO, Cu(OTf)2 and Cu(OAc)2 as promoters in acetic acid and DMF under atmospheric air. They were found to be less effective for this transformation (Table 1, entries 3–9). Next we tried different oxidants such as K2S2O8 (1.0 equiv.) and oxone (1.0 equiv.) in CH3CN at room temperature, but unfortunately the desired product was not formed (Table 1, entries 10 and 11). With equimolar amounts of iodine (Table 1, entries 12–18), this reaction worked well in acetic acid and acetonitrile, but was not effective in other solvents such as toluene, dichloromethane, THF, DMF and ethanol. By simply stirring 1 and 2a with iodine (1.0 equiv.) in acetic acid at room temperature under open air for 2 h, the product was obtained in 92% yield (Table 1, entry 14). When the reaction was carried out with and without oxygen atmospheres, the yield of the reaction in the absence of an air atmosphere was apparently lower, which proved that the O2 air played an energetic role in the process. These optimized results indicate that one equivalent of iodine is necessary for the full conversion of the starting materials. When I2 (0.5 equiv.) was used, only a 47% yield was obtained even after 6 h (Table 1, entry 19).| Entry | Promoter (1.0 equiv.) | Solvent | Temp. | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 2-mercaptobenzimidazole (1.0 equiv.), (E)-1-(4-aryl)-3-(dimethylamino)-prop-2-en-1-one 2 (1.0 equiv.), promoter (1.0 equiv.), oxidant (1.0 equiv.), under open air in 5 mL of solvent. b Isolated yield. c Reagent free reaction. d No reaction. e Chemical formula of oxone is 2KHSO5·KHSO4·K2SO4. | |||||
| 1 | —c | AcOH | RT | 6 | NRd |
| 2 | CuI | AcOH | RT | 12 | 62 |
| 3 | CuI | AcOH | 100 °C | 12 | 57 |
| 4 | CuI | DMF | 100 °C | 12 | 51 |
| 5 | CuCl | DMF | 100 °C | 12 | 20 |
| 6 | CuBr | DMF | 100 °C | 12 | 25 |
| 7 | CuO | DMF | 100 °C | 12 | NR |
| 8 | Cu(OTf)2 | DMF | 100 °C | 12 | 40 |
| 9 | Cu(OAc)2 | DMF | RT | 12 | 48 |
| 10 | K2S2O8 | MeCN | RT | 12 | NR |
| 11 | Oxonee | MeCN | RT | 12 | NR |
| 12 | I2 | Toluene | RT | 6 | 31 |
| 13 | I2 | DCM | RT | 6 | 55 |
| 14 | I2 | AcOH | RT | 2 | 92 |
| 15 | I2 | MeCN | RT | 2 | 81 |
| 16 | I2 | DMF | RT | 6 | 62 |
| 17 | I2 | THF | RT | 6 | 58 |
| 18 | I2 | EtOH | RT | 6 | 38 |
| 19 | I2 (0.5) | AcOH | RT | 6 | 47 |
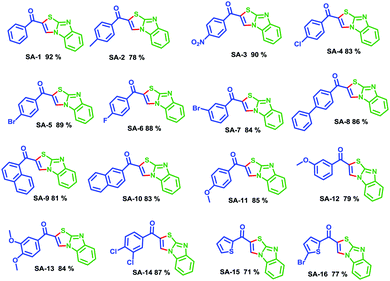
With the optimized conditions in hand, we extended the scope of the reaction using electron-withdrawing and electron-donating substituents in phenyl rings, affording the corresponding products in moderate to good yields (Scheme 2, 78–92%; SA-1–14). The optimized conditions were also successfully applied to hetero aryl enaminones, which generated the corresponding heterocyclic substituted products in moderate yields (71 and 77%; SA-15 and 16), respectively. In order to understand the mechanism of this reaction, the radical trapping experiments were tested (Scheme 3). 2,2,6,6-Tetramethylpiperidinyloxy (TEMPO) was used as a radical scavenger. In the presence of TEMPO, SA-1 was observed in good yield (89%) and this result indicates that radical intermediates were not involved in this reaction (Table 2).24
| a Isolated yield. |
|---|
|
The structures of all the synthesized compounds were confirmed using 1H NMR, 13C NMR, ESI-MASS and HRMS. The structures of compounds SA-2 and SA-6 were undoubtedly confirmed by X-ray diffraction analysis.25 On the basis of the above results and literature reports, a tentative mechanism is proposed for the formation of benzo[4,5]imidazo[2,1-b]thiazole (SA-1–16) as depicted in Scheme 4. Initially the addition reaction between enaminones 2 and iodine leads to the formation of intermediate A.26 The tautomeric thiol 1A under acidic conditions undergoes a nucleophilic substitution reaction affording intermediate Bvia the removal of the HI molecule. This is followed by an intramolecular cyclization giving C, and finally the removal of N,N-dimethylamine under acidic conditions leads to the formation of the desired product benzo[4,5]imidazo[2,1-b]thiazole.
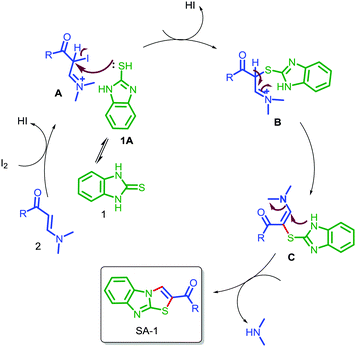 | ||
| Scheme 4 Proposed mechanism for the formation of benzo[4,5]imidazo[2,1-b]thiazol-3-yl(phenyl)methanone. | ||
Conclusions
We have developed a mild, novel and efficient iodine-mediated oxidative C–N and C–S bond formation reaction for the synthesis of substituted benzo[4,5]imidazo[2,1-b]thiazoles. The reaction is highly selective, metal and base free and has a short reaction time.Experimental section
General methods
The melting points were measured in open capillary tubes and are uncorrected. The 1H and 13C NMR spectra were recorded on a Bruker (Avance) 300 MHz NMR instrument using TMS as the internal standard and either CDCl3 or DMSO-d6 as the solvent. The chemical shifts are given in parts per million (δ-scale) and the coupling constants are given in Hertz (Hz). Silica gel-G plates (Merck) were used for thin layer chromatography (TLC) analysis with a mixture of petroleum ether (60–80 °C) and ethyl acetate as the eluent. The single crystal X-ray data were collected on a BRUKER GADDS X-ray (three-circle) diffractometer under Mo Ka (k = 1.5418 Å) irradiation. Elemental analyses were performed on a vario EL III CHNS elemental analyzer. Mass spectra were recorded in a LCQ Fleet mass spectrometer (Thermo Fisher Instruments Limited, USA). Electrospray ionization mass spectrometry (ESI-MS) analysis was performed in the positive ion and negative ion modes using liquid chromatography–ion trap mass spectrometry.General experimental procedure for benzo[4,5]imidazo[2,1-b]thiazol-3-yl(phenyl)methanone SA-1–16
A mixture of 2-mercaptobenzimidazole 1 (1.0 equiv.), (E)-1-(4-aryl)-3-(dimethylamino)-prop-2-en-1-one 2 (1.0 equiv.), and iodine (1.0 equiv.) was added to 5 mL of acetic acid under open air at room temperature for 2 h. The completion of the reaction was monitored using TLC. After completion of the reaction, the reaction mixture was poured into water and work up with sodium thiosulfate and the solution was extracted with ethyl acetate. The combined organic phase was dried over Na2SO4 and then the solvent was removed. The crude sample was purified using column chromatography.Acknowledgements
S. A. gratefully acknowledges UGC-RGNF for the Research Fellowship. We thank the DST, New Delhi, for assistance under the IRHPA program for the NMR facility.Notes and references
- (a) X. Chen, K. M. D. Engle, H. Wang and J. Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094–5115 CrossRef CAS PubMed; (b) C. L. Sun, B. J. Li and Z. J. Shi, Chem. Commun., 2010, 46, 677–685 RSC; (c) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879–5918 CrossRef CAS PubMed; (d) S. A. Girard, T. Knauber and C. J. Li, Angew. Chem., Int. Ed., 2014, 53, 74–100 CrossRef CAS PubMed; (e) J. Zhang, H. Chen, C. Lin, Z. Liu, C. Wang and Y. Zhang, J. Am. Chem. Soc., 2015, 137, 12990–12996 CrossRef CAS PubMed.
- (a) X. X. Guo, D. W. Gu, Z. Wu and W. Zhang, Chem. Rev., 2015, 115, 1622–1651 CrossRef CAS PubMed; (b) D. H. Wang, K. M. Engle, B. F. Shi and J. Q Yu, Science, 2010, 327, 315–319 CrossRef CAS PubMed; (c) R. Giri, B. F. Shi, K. M. Engle, N. Maugel and J. Q. Yu, Chem. Soc. Rev., 2009, 38, 3242–3272 RSC; (d) J. F. Hartwig, Chem. Soc. Rev., 2011, 40, 1992–2002 RSC; (e) C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3464–3484 RSC; (f) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726–11743 CrossRef CAS PubMed; (g) J. W. Delord, T. Dröge, F. Liu and F. Glorius, Chem. Soc. Rev., 2011, 40, 4740–4761 RSC.
- (a) R. K. Kumar, S. Manna, D. Mahesh, D. Sar and T. Punniyamurthy, Asian J. Org. Chem., 2013, 2, 843–847 CrossRef CAS; (b) Y. Chi, W. X. Zhang and Z. Feng, Org. Lett., 2014, 16, 6274–6277 CrossRef CAS PubMed; (c) M. A. Omar, W. Frey, J. Conrad and U. J. Beifuss, J. Org. Chem., 2014, 79, 10367–10377 CrossRef CAS PubMed; (d) W. Zhao, P. Xie, Z. Bian, A. Zhou, H. Ge, M. Zhang, Y. Ding and L. Zheng, J. Org. Chem., 2015, 80, 9167–9175 CrossRef CAS PubMed; (e) W. Yu, Y. Du and K. Zhao, Org. Lett., 2009, 11, 2417–2420 CrossRef CAS PubMed.
- (a) M. Lamani and K. R. Prabhu, J. Org. Chem., 2011, 76, 7938–7944 CrossRef CAS PubMed; (b) X. Wu, Q. Gao, S. Liu and A. Wu, Org. Lett., 2014, 16, 4582–4585 CrossRef PubMed; (c) Y. Xie, J. Wu, X. Che, Y. Chen, H. Huang and G. J. Deng, Green Chem., 2016, 18, 667–671 RSC.
- (a) X. Kang, R. Yan, G. Yu, X. Pang, X. Liu, X. Li, L. Xiang and G. Huang, J. Org. Chem., 2014, 79, 10605–10610 CrossRef CAS PubMed; (b) X. Zhao, T. Li, L. Zhang and K. Lu, Org. Biomol. Chem., 2016, 14, 1131–1137 RSC.
- Z. He, H. Li and Z. Li, J. Org. Chem., 2010, 75, 4636–4639 CrossRef CAS PubMed.
- M. Palkar, M. Noolvi, R. Sankangoud and V. Maddi, Arch. Pharm. Chem. Life Sci., 2010, 343, 353–359 CrossRef CAS PubMed.
- (a) A. Andreani, S. Burnelli, M. Granaiola and A. Leoni, J. Med. Chem., 2008, 51, 7508–7513 CrossRef CAS PubMed; (b) A. Furlan, F. Colombo, A. Kover and N. Issaly, Eur. J. Med. Chem., 2012, 47, 239–247 CrossRef CAS PubMed; (c) J. H. Park, M. I. El-Gamal, Y. S. Lee and G. H. Oh, Eur. J. Med. Chem., 2011, 46, 5769–5777 CrossRef CAS PubMed; (d) T. U. Mayer, T. M. Kapoor, S. J. Haggarty, R. W. King, S. L. Schreiber and T. J. Mitchison, Science, 1999, 286, 971–974 CrossRef CAS PubMed.
- C. B. Vu, J. E. Bemis, J. S. Disch, P. Y. Ng, J. J. Nunes, J. C. Milne, D. P. Carney, A. V. Lynch, J. J. Smith, S. Lavu, P. D. Lambert, D. J. Gagne, M. R. Jirousek, S. Schenk, J. M. Olefsky and R. B. Perni, J. Med. Chem., 2009, 52, 1275–1283 CrossRef CAS PubMed.
- B. Tozkoparan, M. Ertan, P. Kelicen and R. Demirdamar, Farmaco, 1999, 54, 588–593 CrossRef CAS PubMed.
- G. C. Moraski, L. D. Markley, M. Chang, S. Cho, S. G. Franzblau, C. H. Hwang, H. Boshoff and M. Miller, J. Bioorg. Med. Chem., 2012, 20, 2214–2220 CrossRef CAS PubMed.
- A. Locatelli, S. Cosconati, M. Micucci, A. Leoni, L. Marinelli, A. Bedini, P. Loan, S. M. Spampinato, E. Novellino, A. Chiarini and R. Budriesi, J. Med. Chem., 2013, 56, 3866–3877 CrossRef CAS PubMed.
- N. Pietrancosta, A. Moumen, R. Dono, P. Lingor, V. Planchamp, F. Lamballe, M. Bahr, J. L. Kraus and F. Maina, J. Med. Chem., 2006, 49, 3645–3652 CrossRef CAS PubMed.
- T. Mase, H. Arima, K. Tomioka, T. Yamada and K. Murase, J. Med. Chem., 1986, 29, 386–394 CrossRef CAS PubMed.
- G. Huang, H. Sun, X. Qiu, C. Jin, C. Lin, Y. Shen, J. Jiang and L. Wang, Org. Lett., 2011, 13(19), 5224–5227 CrossRef CAS PubMed.
- M. M. Heravi, A. Keivanloo, M. Rahimizadeh, M. Bakavoli and M. Ghassemzadeh, Tetrahedron Lett., 2004, 45, 5747–5751 CrossRef CAS.
- M. Ochiai and N. Tada, Chem. Commun., 2005, 5083–5085 RSC.
- H. Xu, Y. Zhang, J. Huang and W. Chen, Org. Lett., 2010, 12, 3704–3707 CrossRef CAS PubMed.
- D. Xiao, L. Han, Q. Sun, Q. Chen, N. Gong, Y. Lv, F. Suzenet, G. Guillaumet, T. Cheng and R. Li, RSC Adv., 2012, 2, 5054–5057 RSC.
- L. Veltri, G. Grasso, R. Rizzi, R. Mancuso and B. Gabriele, Asian J. Org. Chem., 2016, 5, 560–567 CrossRef CAS.
- (a) B. Stanovnik and J. Svete, Chem. Rev., 2004, 104, 2433–2480 CrossRef CAS PubMed; (b) S. Muthusaravanan, C. Sasikumar, B. Devi Bala and S. Perumal, Green Chem., 2014, 16, 1297–1304 RSC; (c) J. Yang, C. Wang, X. Xie, H. Li and Y. Li, Eur. J. Org. Chem., 2010, 4189–4193 CrossRef CAS.
- (a) S. Kantevari, S. R. Patpi, B. Sridhar, P. Yogeeswari and H. Sriram, Bioorg. Med. Chem. Lett., 2011, 21, 1214–1217 CrossRef CAS PubMed; (b) S. A. Patil, P. A. Medina, D. G. Flores, J. K. Vohs, S. Dever and L. W. Pineda, Synth. Commun., 2013, 43, 2349–2352 CrossRef CAS; (c) K. Goutham, N. S. V. M. Rao Mangina, S. Suresh, P. Raghavaiah and G. V. Karunakar, Org. Biomol. Chem., 2014, 12, 2869–2873 RSC.
- (a) S. Ambethkar, V. Padmini and N. Bhuvanesh, J. Adv. Res., 2015, 6, 975–985 CrossRef CAS PubMed; (b) R. Sribalan, A. Lavanya, M. Kirubavathi and V. Padmini, J. Saudi Chem. Soc., 2016 DOI:10.1016/J.JSCS.2016.03.004; (c) S. Ambethkar, V. Padmini and N. Bhuvanesh, New J. Chem., 2016, 40, 4705–4709 RSC; (d) I. Dhinakaran, V. Padmini and N. Bhuvanesh, ACS Comb. Sci., 2016, 18, 236–242 CrossRef CAS PubMed.
- (a) Y. Yan, Y. Xu, B. Niu, H. Xie and Y. Liu, J. Org. Chem., 2015, 80, 5581–5587 CrossRef CAS PubMed; (b) V. K. Rao, P. Kaswan, G. M. Shelke, A. Ryan, M. Jha and A. Kumar, Synthesis, 2015, 3990–3996 CAS.
- CCDC 1444119 (SA-2) and 1444118 (SA-6).
- J. Safari, Z. A. Jazini, Z. Zarnegar and M. Sadeghi, Catal. Commun., 2005, 7, 763 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H- and 13C-NMR spectra of all compounds, ESI-MS spectra for the selected compounds and X-ray diffraction data for compounds SA-2 and SA-6. CCDC 1444119 and 1444118. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6nj02102f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |