Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Transcriptome profile of yeast reveals the essential role of PMA2 and uncharacterized gene YBR056W-A (MNC1) in adaptation to toxic manganese concentration†
N.
Andreeva
a,
E.
Kulakovskaya
a,
A.
Zvonarev
a,
A.
Penin
bcde,
I.
Eliseeva
f,
A.
Teterina
g,
A.
Lando
gh,
I. V.
Kulakovskiy
*gi and
T.
Kulakovskaya
 *a
*a
aSkryabin Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, pr. Nauki 5, Pushchino, 142290, Russia. E-mail: alla@ibpm.pushchino.ru
bInstitute for Information Transmission Problems, Russian Academy of Sciences, Moscow, 127051, Russia
cA. N. Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, Moscow, 119991, Russia
dLaboratory of Extreme Biology, Institute of Fundamental Biology and Medicine, Kazan Federal University, Kazan, 420012, Russia
eFaculty of Biology, Lomonosov Moscow State University, Moscow, 119991, Russia
fGroup of Protein Biosynthesis Regulation, Institute of Protein Research, Institutskaya 4, Pushchino, 142290, Russia
gVavilov Institute of General Genetics, Russian Academy of Sciences, Gubkina 3, Moscow, GSP-1, 119991, Russia
hMoscow Institute of Physics and Technology (State University), Institutskiy per. 9, Dolgoprudny, Moscow Region 141700, Russia
iEngelhardt Institute of Molecular Biology, Russian Academy of Sciences, Vavilova 32, Moscow, GSP-1, 119991, Russia. E-mail: ivan.kulakovskiy@gmail.com
First published on 9th January 2017
Abstract
Adaptation of S. cerevisiae to toxic concentrations of manganese provides a physiological model of heavy metal homeostasis. Transcriptome analysis of adapted yeast cells reveals upregulation of cell wall and plasma membrane proteins including membrane transporters. The gene expression in adapted cells differs from that of cells under short-term toxic metal stress. Among the most significantly upregulated genes are PMA2, encoding an ortholog of Pma1 H+-ATPase of the plasma membrane, and YBR056W-A, encoding a putative membrane protein Mnc1 that belongs to the CYSTM family and presumably chelates manganese at the cell surface. We demonstrate that these genes are essential for the adaptation to toxic manganese concentration and propose an extended scheme of manganese detoxification in yeast.
Significance to metallomicsYeast provides a unique model to study manganese detoxication, which is important to understand heavy metal ion homeostasis in eukaryotes. A whole-transcriptome analysis of manganese-adapted yeast revealed multiple upregulated genes. Among the top activated genes were PMA2, a rarely expressed H+-ATPase of the cytoplasmic membrane, and YBR056W-A, encoding an uncharacterized protein, a putative member of the CYSTM family. The null mutants demonstrated reduced viability at high manganese ion concentrations. We hypothesize that Pma2 is necessary for cytoplasmic membrane energization and YBR056W-A (Mnc1) chelates manganese at the cell surface and thus participates in manganese detoxication. |
Introduction
Metal cations are common components of the environment and are involved in multiple processes in living cells as enzyme cofactors and metabolism regulators. For example, magnesium fluxes across the plasma membrane regulate timekeeping and energy balance1 and potassium and sodium gradients are essential for cell viability.2 Yeast is an exclusive model to study the adaptation of eukaryotic cells to toxic heavy metals.3–6 Multiple signaling pathways were found to be responsible for yeast viability under an excess of heavy metals. Many genes essential for survival in the presence of copper, silver, zinc, cadmium, mercury, and chromium were previously identified in Saccharomyces cerevisiae.7 The mechanisms of heavy metal tolerance in yeast include environmental sensing, sulfur and glutathione metabolism, vacuolar and endosomal transport and sorting.4 The pathways involved in the Cd2+ tolerance of Schizosaccharomyces pombe included sulfate assimilation, phytochelatin synthesis and transport, ubiquinone biosynthesis, stress signaling, cell wall biosynthesis and cell morphology, gene expression and chromatin remodeling, vacuole function, and intracellular transport of macromolecules.8Manganese is an essential trace element in living cells; its ions are cofactors of many enzymes including oxidases, dehydrogenases, DNA and RNA polymerases, and sugar transferases.9 In humans, disorder of manganese homeostasis is known for neurotoxicity.10,11 High concentrations of manganese are toxic for yeast. Manganese homeostasis in yeast involves transport proteins for Mn2+ uptake, sequestration and excretion; they are located in the plasma membrane, Golgi, vacuolar and mitochondrial membranes.3,12,13 Yeast possesses transporter Smf1, which is localized at the cell surface, and intracellular transporter Smf2, which is localized mainly in intracellular Golgi like vesicles.13 Smf1 also participates in the oxidative stress response.13 The phosphate transporter of the yeast plasma membrane, PHO84, is responsible for Mn2+ uptake via manganese–phosphate complexes.13 Disruption of the PHO84 gene results in a manganese-resistant phenotype.14 This resistance is associated with the inability of yeast cells to take up large amounts of Mn2+. The PHO80 mutants of S. cerevisiae are defective in phosphate uptake, storage and metabolism, and exhibit a wide range of defects in metal homeostasis.15
The Golgi manganese transporter Pmr1p is a P-type calcium and manganese transporting ATPase that transports these ions from the cytoplasm into Golgi lumen.13 This protein participates in detoxification of excess manganese via excretion by the secretory pathway. The vacuolar manganese transporter Ccc1 is responsible for manganese sequestration in vacuoles.16
The sufficient manganese concentration for S. cerevisiae growth is in the range of 0.01–0.1 mM.3,14 Inhibition of the growth of S. cerevisiae was observed at 0.5 mM Mn2+.17 Recently, we have demonstrated the ability of S. cerevisiae to adapt to toxic Mn2+ concentrations (2–5 mM) after an unusually long lag phase (4–5 days).18 The adaptation was accompanied by the enlargement of vacuoles and whole cells, and with the drastic increase in the content and chain length of acid soluble polyphosphate, which probably forms complexes with manganese ions.18,19 If re-inoculated into a fresh medium with Mn2+, the adapted cells had no growth delay.18 Such adaptation is a useful model to study yeast tolerance to toxic manganese concentrations.
In this study, we have used RNA-Seq to analyze the whole transcriptome of S. cerevisiae adapted to the toxic concentration of Mn2+.
Materials and methods
CRY strain and growth conditions
The strain CRY MATa (Δade2 Δhis3 Δleu2 Δtrp1 Δura3) was kindly provided by N. Rao and A. Kornberg.20 This strain was used earlier as a model for manganese adaptation.18 The cells of this strain were used for transcriptome analysis (two technical replicates of the same cultivation), enzyme activity assay, and qPCR verification of differential gene expression.The cells were cultivated in YPD (2% glucose, 2% peptone, 1% yeast extract) at 29 °C and 145 rpm for 18 hours in the control medium and for 120 hours in manganese-rich medium (stationary growth stage). Growth curves for CRY are shown in the ESI,† Fig. S1. The CRY strain growth curve and cell morphology change in the presence of 2.5 to 5.0 mM Mn2+.18 MnSO4 was added to a final concentration of 2.5 mM. In the control YPD medium, the Mn2+ concentration was estimated to be 0.0036 mM. The cells were harvested at 5000g for 20 min, washed with sterile distilled water, and used for analysis.
Mutant strains
Mutant strains were used to assess the involvement of particular genes in manganese adaptation. The yeast parental strain BY4743, Yeast HomDip Knock Out Strain YBR056W-A (Δybr056-a), Yeast HomDip Knock Out Strain YPL036W (Δpma2), and Yeast HomDip Knock Out Strain YMR303C (Δadh2) were obtained from Yeast Knockout Collection (YKO, Dharmacon) and grown in standard YPD medium or with the addition of 2.5 mM MnSO4.RNA extraction and sequencing
After growing to A600 of 16 (control) and 12 (manganese, see the ESI,† Fig. S1), the cells were washed twice with distilled water at 0 °C and centrifuged after each washing. This step was applied to both control and manganese-adapted cells. Washing was necessary because without it RNA degradation in yeast samples grown in manganese medium prevented sequencing library construction. The resulting samples were placed in RNALater, frozen and used for RNA isolation. Total RNA was extracted using the RNEasy Mini kit (Qiagen), with the addition of the Plant RNA Isolation aid reagent (Ambion) to lysis buffer RLT. Libraries were prepared using the TruSeq RNA sample preparation kit (Illumina) and sequenced using a HiSeq2000 instrument with 50 nt read length.Enzyme activity assay
For assay of enzyme activities, each sample of biomass was frozen at −70 °C, and the cells were broken by French-press (three biological replicates). The homogenate was suspended in 10 mM Tris-HCl, pH 7.0, with 0.5 mM PMSF. The homogenate was centrifuged at 3000g for 5 min and then the supernatant was centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 60 min. The supernatant (cellular extract) was used for the assay of alcohol dehydrogenase activity; the pellet (crude membrane fraction) was suspended in 50 mM MES-Na, pH 6.0, and used for the assay of the plasma membrane and mitochondrial ATPase activities. The alcohol dehydrogenase activity was measured as described in ref. 21. The activities of plasma membrane ATPase and mitochondrial ATPase were estimated by Pi release as described in ref. 22. The reaction was carried out in 50 mM MES-Na, pH 6.0, containing 6 mM ATP and 4 mM MgSO4 or MnSO4 at 30 °C in the case of plasma membrane ATPase. The activity was measured in the absence and presence of 0.1 mM orthovanadate, a specific inhibitor of the plasma membrane ATPase of yeast.22 The vanadate-sensitive activity was taken as the activity of plasma membrane ATPase. The reaction was carried out in 50 mM Tris-HCl, pH 8.5, containing 2.5 mM ATP and 2.5 mM MgSO4 at 30 °C in the case of mitochondrial ATPase. As a specific inhibitor, 10 mM of NaN3 was added. In both cases, the reagent for the Pi assay was added as a stop solution.23 All assays were repeated in triplicate and the average values with standard deviations were computed with MS Excel.
000g for 60 min. The supernatant (cellular extract) was used for the assay of alcohol dehydrogenase activity; the pellet (crude membrane fraction) was suspended in 50 mM MES-Na, pH 6.0, and used for the assay of the plasma membrane and mitochondrial ATPase activities. The alcohol dehydrogenase activity was measured as described in ref. 21. The activities of plasma membrane ATPase and mitochondrial ATPase were estimated by Pi release as described in ref. 22. The reaction was carried out in 50 mM MES-Na, pH 6.0, containing 6 mM ATP and 4 mM MgSO4 or MnSO4 at 30 °C in the case of plasma membrane ATPase. The activity was measured in the absence and presence of 0.1 mM orthovanadate, a specific inhibitor of the plasma membrane ATPase of yeast.22 The vanadate-sensitive activity was taken as the activity of plasma membrane ATPase. The reaction was carried out in 50 mM Tris-HCl, pH 8.5, containing 2.5 mM ATP and 2.5 mM MgSO4 at 30 °C in the case of mitochondrial ATPase. As a specific inhibitor, 10 mM of NaN3 was added. In both cases, the reagent for the Pi assay was added as a stop solution.23 All assays were repeated in triplicate and the average values with standard deviations were computed with MS Excel.
Fluorescence and light microscopy
Living and dead cells were revealed using the LIVE/DEAD Fungal Light Yeast Viability Kit (Molecular Probes Inc., USA) according to the official manual. Yeast cultures were stained without washing and incubated with a stain reagent for 15 min at 37 °C. The samples were examined under the fluorescence microscope AXIO Imager A1 ZEISS (Germany) with a filter kit 56 (ZEISS) at a wavelength of 450–500 nm (excitation) and 600–650 nm (emission). The cell concentration was estimated by light microscopy in a standard counting chamber. All assays were repeated in triplicate and the average values with standard deviations were computed with MS Excel.Processing of RNA-Seq data
The raw reads were processed as follows: trimming was performed with cutadapt24 and sickle,25 mapping to the S288C reference genome26 was performed with TopHat,27 HTSeq28 was used for read counting, and R environment and edgeR29 were used to estimate differential gene expression. Overall statistics and the complete data on differential gene expression are provided in the ESI,† Table S1.The raw and processed data are deposited in GEO (accession number GSE85109).
qPCR assay
The biomass samples were frozen at −70 °C and broken by French press (two biological replicates). Total RNA was isolated by TRIzol LS Reagent (Thermo Scientific). Genomic DNA was removed by treating RNA samples with the RNase-free DNase I (Thermo Scientific). cDNA was obtained using the Maxima H Minus First Strand cDNA Synthesis Kit (Thermo Scientific). qPCR was performed using the qPCRmix-HS SYBR + LowROX kit (Evrogen) and the DTlite Real-Time PCR System (DNA Technology).Spot test assay
A spot test was performed on YPD medium supplemented with 4% agar. 0.005 ml of cell suspension was applied per spot with the cell concentrations from 2 × 106 to 2 × 104 cell ml−1. Petri dishes were incubated at 28 °C for 24 h (control) and 44 h (5 mM Mn2+, 2.5 mM used in liquid medium were not toxic in solid medium).Results
The cells of S. cerevisiae adapted to 2.5 mM of manganese after a prolonged lag-phase are characterized by specific changes in morphology.18 To find out the genes involved in manganese adaptation we performed transcriptome analysis of the control and adapted cells in the early stationary growth stage.Using the arbitrary threshold of FDR < 0.05 and requiring a two fold difference (|log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 fold change| > 1) for gene expression between the experiment and the control, we identified 609 (537) upregulated (downregulated) genes. The GO-enrichment analysis performed with YeastMine30 revealed downregulation of various cellular biosynthesis processes (including translation and ribosome assembly), which agrees with the observed decrease in the growth rate (see the ESI,† Fig. S1). Upregulated genes were enriched in GO-terms associated with the cell wall and membrane assembly and various transporter activities (including ion transporters, see the ESI,† Table S2).
2 fold change| > 1) for gene expression between the experiment and the control, we identified 609 (537) upregulated (downregulated) genes. The GO-enrichment analysis performed with YeastMine30 revealed downregulation of various cellular biosynthesis processes (including translation and ribosome assembly), which agrees with the observed decrease in the growth rate (see the ESI,† Fig. S1). Upregulated genes were enriched in GO-terms associated with the cell wall and membrane assembly and various transporter activities (including ion transporters, see the ESI,† Table S2).
Manganese adaptation upregulates genes not involved in known metal stress responses
We took a closer look at the top 10 upregulated genes with the lowest FDR and 10–20-fold increase in expression under manganese adaptation (Table 1). Some genes (such as PHO89 encoding the plasma membrane Na+/Pi cotransporter31) are consistently upregulated in various metal stresses.7,32 Upregulation of other genes is detected specifically in manganese adaptation: HXT2 (a high-affinity glucose transporter), YNR064C (epoxide hydrolase), POX1 (Fatty-acyl coenzyme A oxidase), PMA2 (the ortholog of PMA1 encoding the plasma membrane H+-ATPase that is rarely expressed, e.g. in diauxic growth33), and YBR056W-A gene encoding an uncharacterized protein. Adaptation-specific genes YBR056W-A and PMA2 were selected for detailed analysis and verification. Additionally, in further analysis we considered ADH2 encoding glucose-repressed alcohol dehydrogenase, characteristic of the stationary growth stage34 and upregulated in other metal stresses.| Gene systematic name (according to the Saccharomyces Genome database) | Gene name | Upregulated in | ||
|---|---|---|---|---|
| manganese adaptation | Short-term manganese exposure | Other metal stresses | ||
| YMR011W | HXT2 | + | − | − |
| YMR303C | ADH2 | + | − | + |
| YBR056W-A | (Uncharacterized protein) MNC1 | + | − | − |
| YNR064C | (Epoxide hydrolase) | + | − | − |
| YOR385W | (DIA1 homolog) | + | + | + |
| YMR316W | DIA1 | + | + | + |
| YBR296C | PHO89 | + | + | + |
| YPL036W | PMA2 | + | − | − |
| YGL205W | POX1 | + | − | − |
First of all we focused particular attention on the YBR056W-A gene encoding the previously uncharacterized protein. This gene is translated35 and encodes a peptide (66 a.a.) that bears a notable similarity to cysteine-rich TM module stress tolerance domain36 (CDD37 Blast E-value ∼2 × 10−15, see also the ESI,† Fig. S2 for a multiple sequence alignment). The members of the CYSTM family were reported to play a role in stress responses, in particular, these proteins prevent metal uptake into plant cells.38 We verified the YBR056W-A differential expression by qPCR (see the ESI,† Fig. S3), which confirmed the upregulation in the manganese-adapted yeast. Based on this evidence, we suggest MNC1 (manganese-chelating protein 1) as a gene symbol for YBR056W-A.
Enzyme activity assay confirms differential gene expression
To provide an independent validation of RNA-Seq differential expression, we compared the activities of alcohol dehydrogenase and H+-ATPases in control and manganese-adapted cells.The activity of mitochondrial ATPase in manganese-adapted cells was lower compared to the control cells (Table 2). It agrees with the down-regulation (FDR < 0.05) of most of the genes encoding F1Fo-ATPase subunits (ESI,† Table S1). The gene expression of PMA1 encoding the major H+-ATPase was unchanged under adaptation, while the PMA2 ortholog was significantly upregulated. However, the activity of plasma-membrane ATPase in manganese-adapted cells was lower compared to the control cells (Table 2). It was shown that Pma2 is more active in the presence of manganese than Pma1.39 Probably, the upregulation of Pma2 maintains the transmembrane potential at the plasma membrane under manganese excess.
| Enzyme activity, E/mg protein | Control cells | Mn2+ adapted cells |
|---|---|---|
| Alcohol dehydrogenase | 4.9 ± 2.0 | 11.7 ± 4.0 |
| Mitochondrial ATPase | 0.64 ± 0.2 | 0.2 ± 0.05 |
| Plasma membrane ATPase | 0.32 ± 0.02 | 0.21 ± 0.09 |
The activity of alcohol dehydrogenase in the cell-free extract of manganese-adapted cells was higher compared to the control cells (Table 2). However, this increase was less marked compared to the gene expression data (2 times versus more than 10 times). Probably, this difference is due to a specific property of Adh2: the enzyme contains a Zn2+ ion in the active center, which is replaced by a Mn2+ ion under manganese excess lowering the stability of the enzyme.40
Mutant strains exhibit different adaptability to toxic manganese concentrations
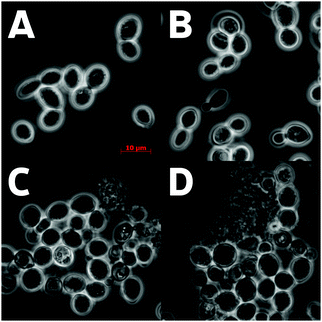
The adaptation to manganese was studied in three BY4743 mutant strains: Δybr056w-a, Δpma2, and Δadh2. All strains grew similarly in YPD medium; in the presence of 2.5 mM manganese, the growth of all strains was inhibited (ESI,† Fig. S4). The adaptation of BY4743 cells to manganese was similar to that of CRY cells with decreased growth rate and reduced optical density at the stationary phase compared to growth in YPD medium (ESI,† Fig. S1 and S4). The increase in the cells' sizes in manganese-medium compared to the control medium was also observed (see Fig. 1A and C).The BY4743 and all the mutant strains had similar cell concentrations and culture densities in control growth (ESI,† Fig. S4). However, notable differences were found for growth in manganese-rich medium: (1) the culture density of the Δadh2 mutant was notably higher than that of the three other strains including BY4743, but (2) the cell concentrations of the Δybr056w-a and Δpma2 mutants were notably lower than of the parent strain and of the Δadh2 mutant (ESI,† Fig. S4).
The light microscopy of the Δybr056w-a and Δpma2 cultures revealed large-scale cell lysis in the stationary phase in manganese-rich medium (Fig. 2). Indeed, the staining using the LIVE/DEAD Yeast Viability Kit showed that the cell cultures of Δybr056w-a and Δpma2 contained multiple dead and lysed cells (Fig. 1, 2 and Table 3). Probably, the cell lysis products enhance light scattering resulting in comparably high optical density of these cell cultures. The presence of living cells in the cultures of these mutants is probably due the fact that the cells, which have not yet accumulated a toxic amount of manganese, preserved the budding ability (Fig. 1).
| Strain | Dead cells, % | |
|---|---|---|
| Control YPD | YPD, 2.5 mM MnSO4 | |
| BY4743, parent strain | 0 | 12 |
| Δybr056w-a | 0 | 52 |
| Δypl036w (Δpma2) | 0 | 62 |
| Δymr303c (Δadh2) | 0 | 18 |
The Δadh2 mutant demonstrated the most resistance to manganese (ESI,† Fig. S4 and Table 3); in particular, it did not demonstrate cell lysis in the manganese-rich medium (Fig. 2). The stability of the Adh2 enzyme drastically decreases under excess manganese.40 Probably, observed upregulation of ADH2 can be a result of the feedback mechanism to compensate for the rapid inactivation of the enzyme. It is not clear why the Δadh2 mutant is more manganese-resistant than the parent strain, but this was confirmed by the Spot test (ESI,† Fig. S5).
However, the same test failed to distinguish Δybr056w-a and Δpma2 from the parent strain when grown on manganese-rich medium; this was also the case for the culture density test (ESI,† Fig. S4).
All in all, it seems Mn2+ excess does not prevent budding of Δybr056w-a and Δpma2, but induces cell lysis in later stages of the cell cycle. In particular, this explains the limitation of the culture density test and the Spot test41 to determine the growth differences in manganese-rich medium.
Discussion
We compared the set of genes upregulated in manganese adaptation with the sets of genes upregulated upon various metal stresses found in previous microarray experiments.7,32 There was only a small overlap between sets of upregulated genes comparing adaptation and short-term exposure32 to Mn2+: 570 of the 609 upregulated genes were specific to adaptation (not upregulated in short-term manganese exposure) and 419 of the 609 adaptation-upregulated genes were not upregulated in any other metal stresses.7,32 In particular, YeastMine30 analysis of gene ontology for genes unique to short-term manganese stress shows activation of general biosynthesis pathways (primarily amino-acid and organic acid synthesis). In contrast, genes unique for adaptation are enriched with sugar transporters and cell wall and membrane proteins. Thus, yeast manganese adaptation drastically differs from short-term stress response in terms of gene expression patterns. This highlights the specific state of adapted cells, which pertain morphology highly deviating both from control cells and cells under stress response.18Multiple proteins participate in manganese homeostasis in S. cerevisiae.5,13 Based on our findings we propose several key additions to the existing model of manganese detoxication in yeast cells (Fig. 3). The adaptation of yeast cells to toxic concentrations of manganese ions involves, in particular, transport systems responsible for manganese compartmentalization, systems of phosphorus transport and polyphosphate accumulation, as well as other membrane proteins.
 | ||
| Fig. 3 Scheme of manganese detoxication in S. cerevisiae and proposed role of YBR056W (Mnc1) and Pma2. | ||
In particular, Smf1 is a known manganese transport protein localized in the plasma membrane and endosomes13 and responsible for manganese ion uptake. On the one hand, its expression does not change upon adaptation (Table 4). This agrees with reported Smf1 stability under manganese exposure13 and high level of manganese uptake in adapted cells.18 On the other hand, the genes of transporters providing Mn2+ compartmentation (Smf2, Ccc1 and Pmr1) are slightly upregulated (passing FDR < 0.05) in manganese adapted cells (Table 4) enhancing manganese sequestration in the endoplasmic reticulum, vacuoles, and Golgi, respectively. Interestingly, Spf1, an important regulator of the manganese transport in ER,42 demonstrates stable expression between growth in control and Mn2+ excess conditions (ESI,† Table S1). Thus, low differential expression of manganese transporters suggests limited involvement of these proteins in adaptation to toxic manganese concentration.
Expression fold change (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
FDR | Gene symbol | Gene function |
|---|---|---|---|
| Polyphosphate metabolism enzymes | |||
| 0.15 | 0.25 | PPN1 | Exo/endopolyphosphatase |
| ↓−0.89 | 6.75 × 10−15 | PPX1 | Exopolyphosphatase |
| −0.04 | 0.8 | DDP1 | Diadenosine and diphosphoinositol polyphosphate phosphohydrolase |
| −0.14 | 0.26 | VTC4 | Polyphosphate synthase |
| PHO pathway members | |||
| ↑0.95 | 3.92 × 10−16 | PHO84 | High-affinity inorganic phosphate (Pi) transporter; also low-affinity manganese transporter |
| ↑0.70 | 6.09 × 10−10 | PHO87 | Low-affinity inorganic phosphate (Pi) transporter |
| ↑3.35 | 1.6 × 10−143 | PHO89 | Plasma membrane Na+/Pi cotransporter |
| ↑1.01 | 2.28 × 10−16 | PHO90 | Low-affinity phosphate transporter |
| Plasma ATPases | |||
| 0.0 | 1 | PMA1 | Plasma membrane H+-ATPase |
| ↑4.72 | 2.29 × 10−145 | PMA2 | Plasma membrane H+-ATPase |
| Manganese transporters | |||
| 0.15 | 0.23 | SMF1 | Cell surface manganese transporter |
| ↑0.63 | 8.3 × 10−08 | SMF2 | ER membrane manganese transporter |
| ↑0.38 | 0.0006 | PMR1 | Golgi manganese transporter |
| ↑0.60 | 3.79 × 10−7 | CCC1 | Vacuolar membrane manganese transporter |
| Upregulated uncharacterized proteins | |||
| ↑5.12 | 5.4 × 10−185 | YBR056W-A (MNC1) | CYSTM-family protein |
| ↑4.55 | 1.08 × 10−181 | YOR385W | Homologous to DIA1P |
Variable upregulation of multiple genes encoding PHO-proteins is also observed in manganese-adapted cells, in particular, there are cytoplasmic membrane phosphate transporters Pho84, Pho87, Pho89 and Pho90. Previously it was reported that Pho84 is responsible for phosphate and manganese uptake under manganese excess.15 It is not clear whether upregulation of Pho84 has any adaptive significance in our conditions or whether it is a side-effect of disturbed phosphorus metabolism. However, upregulation of phosphate transporters explains the increase of phosphate accumulation by manganese adapted cells.18 The Pi excess may increase the synthesis of inorganic polyphosphate by Vtc4 polyphosphate synthase, the activity of which is stimulated by manganese ions. As for polyphosphate hydrolases, no upregulated genes were found, while Ppx1 polyphosphatase was downregulated. Thus, the enhanced Pi uptake and polyphosphate synthesis and the decreased polyphosphate degradation explain the polyphosphate accumulation in manganese adapted cells.18 Probably, the polymers accumulated in all cellular compartments bind manganese and provide an additional way of detoxication.
The enhanced expression of phosphate transporters, as well as other transporter proteins (including hexose transporters, see the ESI,† Table S1), requires an increase of cytoplasmic membrane energization under manganese adaptation. The expression of the major H-ATPase gene, PMA1, was unchanged, while the expression of its ortholog, PMA2, considerably increased. Probably, the increase in the PMA2 expression, which is more active in the presence of manganese than PMA1,39 is necessary for cytoplasmic membrane energization. Cell lysis of the Δpma2 mutant proves the necessity of PMA2 for yeast adaptation to high concentrations of manganese ions. At the same time, the lytic phenotype of the Δpma2 and Δybr056w-a strains (Fig. 2) makes it difficult to study their transcriptomic state and physiological properties under Mn2+ conditions.
The important role of YBR056W-A in manganese detoxification suggests analysis of other CYSTM family members. For example, dimers of CYSTM protein Pcc1 of Arabidopsis thaliana are anchored in the plasma membrane.43 A suggested role of CYSTM proteins in metal homeostasis is as follows: the peculiar arrangement of sulfhydryl groups of the protein within the membrane could alter the redox potential of the membrane or directly chelate metal ions.36
A yeast paralog of YBR056W-A, YDR034W-B44,45 also encodes a short peptide (51 a.a.) and has been previously annotated as CYSTM protein and supposed to be localized in the cell periphery.44,46 However, YDR034W-B did not change its expression under manganese adaptation in this study. Other known members of the CYSTM family were also stably (e.g. YBR016W, YDL012C) or lowly (YDR210W) expressed. Probably they are responsible for chelating other metal ions.
Notably, the microarrays used in the studies5,32 did not include any probes for the YBR056W-A gene. Thus, it is still possible that YBR056W-A is involved not only in adaptation but in the metal stress response as well. The CYSTM family is not fully annotated and we suppose its significance in stress and pathological processes is yet underestimated. For example, human CYSTM1 was recently found as a biomarker of Huntington's disease.47
Conclusions
In this study for the first time we showed the functional relevance of an uncharacterized protein, YBR056W-A, which is essential for manganese detoxication. The proposed role of this CYSTM protein is to bind manganese at the cell surface, suggesting MNC1 (manganese-chelating protein) as a possible standard gene name. Further studies may elucidate other roles of YBR056W-A (Mnc1) and other CYSTM proteins in stress responses.Acknowledgements
We thank Sergey Gladyshev for his help with preliminary bioinformatics analysis and Maria Logacheva for support with high-throughput sequencing. RNA-Seq library preparation and sequencing was supported by Russian science foundation grant #14-50-00029. Bioinformatics analysis of the transcriptome was supported by Russian science foundation grant #14-50-00060.References
- K. A. Feeney, L. L. Hansen, M. Putker, C. Olivares-Yañez, J. Day, L. J. Eades, L. F. Larrondo, N. P. Hoyle, J. S. O'Neill and G. van Ooijen, Nature, 2016, 532, 375–379 CrossRef CAS PubMed.
- D. Canadell and J. Ariño, Adv. Exp. Med. Biol., 2016, vol. 892, pp. 271–289 Search PubMed.
- V. C. Culotta, M. Yang and M. D. Hall, Eukaryotic Cell, 2005, 4, 1159–1165 CrossRef CAS PubMed.
- M. Thorsen, G. G. Perrone, E. Kristiansson, M. Traini, T. Ye, I. W. Dawes, O. Nerman and M. J. Tamás, BMC Genomics, 2009, 10, 105 CrossRef PubMed.
- R. Wysocki and M. J. Tamás, FEMS Microbiol. Rev., 2010, 34, 925–951 CrossRef CAS PubMed.
- S. Rajakumar, C. Ravi and V. Nachiappan, Metallomics, 2016, 8, 453–460 RSC.
- Y. H. Jin, P. E. Dunlap, S. J. McBride, H. Al-Refai, P. R. Bushel and J. H. Freedman, PLoS Genet., 2008, 4, e1000053 Search PubMed.
- P. J. Kennedy, A. A. Vashisht, K.-L. Hoe, D.-U. Kim, H.-O. Park, J. Hayles and P. Russell, Toxicol. Sci., 2008, 106, 124–139 CrossRef CAS PubMed.
- J. Crowley, D. Traynor and D. Weatherburn, in Manganese and its role in biological processes. Met. Ions Biol. Syst., ed. A. Siegel and H. Siegel, 1999, pp. 209–257 Search PubMed.
- G. F. Kwakye, M. M. B. Paoliello, S. Mukhopadhyay, A. B. Bowman and M. Aschner, Int. J. Environ. Res. Public Health, 2015, 12, 7519–7540 CrossRef CAS PubMed.
- S. Bouabid, A. Tinakoua, N. Lakhdar-Ghazal and A. Benazzouz, J. Neurochem., 2016, 136, 677–691 CrossRef CAS PubMed.
- V. C. Culotta and M. J. Daly, Antioxid. Redox Signaling, 2013, 19, 933–944 CrossRef CAS PubMed.
- A. R. Reddi, L. T. Jensen and V. C. Culotta, Chem. Rev., 2009, 109, 4722–4732 CrossRef CAS PubMed.
- L. T. Jensen, M. Ajua-Alemanji and V. C. Culotta, J. Biol. Chem., 2003, 278, 42036–42040 CrossRef CAS PubMed.
- L. Rosenfeld, A. R. Reddi, E. Leung, K. Aranda, L. T. Jensen and V. C. Culotta, J. Biol. Inorg. Chem., 2010, 15, 1051–1062 CrossRef CAS PubMed.
- L. Li, O. S. Chen, D. M. Ward and J. Kaplan, J. Biol. Chem., 2001, 276, 29515–29519 CrossRef CAS PubMed.
- K. J. Blackwell, J. M. Tobin and S. V. Avery, Appl. Microbiol. Biotechnol., 1998, 49, 751–757 CrossRef CAS PubMed.
- N. Andreeva, L. Ryazanova, V. Dmitriev, T. Kulakovskaya and I. Kulaev, FEMS Yeast Res., 2013, 13, 463–470 CrossRef CAS PubMed.
- N. Andreeva, L. Ryazanova, V. Dmitriev, T. Kulakovskaya and I. Kulaev, Folia Microbiol., 2014, 59, 381–389 CrossRef CAS PubMed.
- A. Sethuraman, N. N. Rao and A. Kornberg, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 8542–8547 CrossRef CAS PubMed.
- J. H. Kagi and B. L. Vallee, J. Biol. Chem., 1960, 235, 3188–3192 CAS.
- B. J. Bowman, S. E. Mainzer, K. E. Allen and C. W. Slayman, Biochim. Biophys. Acta, Biomembr., 1978, 512, 13–28 CrossRef CAS.
- T. V Kulakovskaya, N. A. Andreeva, A. V Karpov, I. A. Sidorov and I. S. Kulaev, Biochemistry, 1999, 64, 990–993 Search PubMed.
- M. Martin, EMBnet.journal, 2011, 17, 10 CrossRef.
- N. Joshi and J. Fass, Available at http://https://github.com/najoshi/sickle, 2011.
- S. R. Engel, F. S. Dietrich, D. G. Fisk, G. Binkley, R. Balakrishnan, M. C. Costanzo, S. S. Dwight, B. C. Hitz, K. Karra, R. S. Nash, S. Weng, E. D. Wong, P. Lloyd, M. S. Skrzypek, S. R. Miyasato, M. Simison and J. M. Cherry, G3, 2014, 4, 389–398 CrossRef PubMed.
- C. Trapnell, L. Pachter and S. L. Salzberg, Bioinformatics, 2009, 25, 1105–1111 CrossRef CAS PubMed.
- S. Anders, P. T. Pyl and W. Huber, Bioinformatics, 2014, 31, 166–169 CrossRef PubMed.
- M. D. Robinson, D. J. McCarthy and G. K. Smyth, Bioinformatics, 2010, 26, 139–140 CrossRef CAS PubMed.
- R. Balakrishnan, J. Park, K. Karra, B. C. Hitz, G. Binkley, E. L. Hong, J. Sullivan, G. Micklem and J. M. Cherry, Database, 2012, 2012, bar062 CrossRef PubMed.
- P. Sengottaiyan, J. Petrlova, J. O. Lagerstedt, L. Ruiz-Pavon, M. S. Budamagunta, J. C. Voss and B. L. Persson, Biochem. Biophys. Res. Commun., 2013, 436, 551–556 CrossRef CAS PubMed.
- D. Hosiner, S. Gerber, H. Lichtenberg-Fraté, W. Glaser, C. Schüller and E. Klipp, PLoS One, 2014, 9, e83330 Search PubMed.
- A. R. Fernandes and I. Sá-Correia, Yeast, 2003, 20, 207–219 CrossRef CAS PubMed.
- R. C. Vallari, W. J. Cook, D. C. Audino, M. J. Morgan, D. E. Jensen, A. P. Laudano and C. L. Denis, Mol. Cell. Biol., 1992, 12, 1663–1673 CrossRef CAS PubMed.
- A. M. Michel, G. Fox, A. M. Kiran, C. De Bo, P. B. F. O'Connor, S. M. Heaphy, J. P. A. Mullan, C. A. Donohue, D. G. Higgins and P. V. Baranov, Nucleic Acids Res., 2014, 42, D859–D864 CrossRef CAS PubMed.
- T. M. Venancio and L. Aravind, Bioinformatics, 2010, 26, 149–152 CrossRef CAS PubMed.
- A. Marchler-Bauer, M. K. Derbyshire, N. R. Gonzales, S. Lu, F. Chitsaz, L. Y. Geer, R. C. Geer, J. He, M. Gwadz, D. I. Hurwitz, C. J. Lanczycki, F. Lu, G. H. Marchler, J. S. Song, N. Thanki, Z. Wang, R. A. Yamashita, D. Zhang, C. Zheng and S. H. Bryant, Nucleic Acids Res., 2015, 43, D222–D226 CrossRef PubMed.
- M. Kuramata, S. Masuya, Y. Takahashi, E. Kitagawa, C. Inoue, S. Ishikawa, S. Youssefian and T. Kusano, Plant Cell Physiol., 2009, 50, 106–117 CrossRef CAS PubMed.
- P. Supply, A. Wach and A. Goffeau, J. Biol. Chem., 1993, 268, 19753–19759 CAS.
- P. L. Coleman and H. Weiner, Biochemistry, 1973, 12, 3466–3472 CrossRef CAS PubMed.
- M. Kwolek-Mirek and R. Zadrag-Tecza, FEMS Yeast Res., 2014, 14, 1068–1079 CAS.
- Y. Cohen, M. Megyeri, O. C. W. Chen, G. Condomitti, I. Riezman, U. Loizides-Mangold, A. Abdul-Sada, N. Rimon, H. Riezman, F. M. Platt, A. H. Futerman and M. Schuldiner, PLoS One, 2013, 8, e85519 Search PubMed.
- R. Mir and J. León, PLoS One, 2014, 9, e87216 Search PubMed.
- J. M. Cherry, E. L. Hong, C. Amundsen, R. Balakrishnan, G. Binkley, E. T. Chan, K. R. Christie, M. C. Costanzo, S. S. Dwight, S. R. Engel, D. G. Fisk, J. E. Hirschman, B. C. Hitz, K. Karra, C. J. Krieger, S. R. Miyasato, R. S. Nash, J. Park, M. S. Skrzypek, M. Simison, S. Weng and E. D. Wong, Nucleic Acids Res., 2012, 40, D700–D705 CrossRef CAS PubMed.
- K. P. Byrne and K. H. Wolfe, Genome Res., 2005, 15, 1456–1461 CrossRef CAS PubMed.
- W.-K. Huh, J. V Falvo, L. C. Gerke, A. S. Carroll, R. W. Howson, J. S. Weissman and E. K. O'Shea, Nature, 2003, 425, 686–691 CrossRef CAS PubMed.
- A. Mastrokolias, Y. Ariyurek, J. J. Goeman, E. van Duijn, R. A. C. Roos, R. C. van der Mast, G. B. van Ommen, J. T. den Dunnen, P. A. C. 't Hoen and W. M. C. van Roon-Mom, Eur. J. Hum. Genet., 2015, 23, 1349–1356 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary figures (S1–S5) and supplementary tables (S1 and S2). See DOI: 10.1039/c6mt00210b |
| This journal is © The Royal Society of Chemistry 2017 |