Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Independent responsive behaviour and communication in hydrogel objects†
Ross W.
Jaggers
and
Stefan A. F.
Bon
 *
*
BonLab, Department of Chemistry, University of Warwick, CV4 7AL, UK. E-mail: s.bon@warwick.ac.uk; Web: www.bonlab.info
First published on 21st March 2017
Abstract
In this work, we show the fabrication of soft hydrogel alginate-based objects, namely fibres and beads, that have an individually programmed time delay in their response to a shared environmental stimulus. We utilize the enzyme urease to programme a self-regulated change in pH, which in turn activates the designed response of gel fibre disintegration or a change in gel bead colour. This design allows for independent response behaviour of a collection of bodies in a single closed system, as well as inter-material communication on shorter length scales. The incorporation of responsive time control directly into soft matter objects demonstrates an advance in the field of autonomous materials.
Conceptual insightsAutonomous response mechanisms are vital to the survival of living organisms and play a key role in both biological function and independent behaviour. The design of artificial life, such as neural networks that model the human brain and robotic devices that can perform complex tasks, relies on programmed intelligence so that responses to stimuli are possible. Responsive synthetic materials can translate environmental stimuli into a direct material response, for example thermoresponsive shape change in polymer gels or light-triggered drug release from capsules. Materials that have the ability to moderate their own behaviour over time and selectively respond to their environment, however, display autonomy and more closely resemble those found in nature. In this communication, we present soft hydrogel objects that possess an individually programmed time delay in their response to a shared environmental stimulus. We utilize the enzyme urease to programme a self-regulated change in pH, which in turn activates the designed response of gel disintegration. This design allows for independent response behaviour of a collection of bodies in a single closed system, as well as inter-material communication on shorter length scales. The incorporation of responsive time control directly into soft matter objects demonstrates an advance in the field of autonomous materials. |
Living organisms can exhibit autonomy in their response to an external stimulus, a concept that drives both biological function and independent behaviour.1–3 Some form of intelligence is required to act in this way, and it is this principle that underpins the design of artificial life.4,5 For example, neural networks model the human brain and robotic devices can perform complex tasks.6,7
A key question in material design is “can this complex concept be stripped down to its essence without loss of autonomy?”.
A multitude of materials translate environmental stimuli into direct material responses. Examples include the catalytically driven actuation of colloidal particles,8–10 light-triggered inversion of emulsions11 and triggered change in capsule permeability.12–15 Though simple stimuli-response pathways can generate sophisticated behaviour, control is often dominated by the environmental cues, not the material itself.16
Alternatively, materials that moderate their own behaviour over time and selectively respond to their environments display autonomy and more closely resemble those found in nature.17,18 For example, the oscillatory Belousov–Zhabotinsky reaction has been used as an internal driver and communication tool,19,20 kinetic control has been used to trap supramolecular self-assemblies in metastable states,21–23 and homeostatic control has been demonstrated.24–26 Furthermore, enzymes have been used to programme the time-domain, as in the case of pH-responsive self-assemblies that utilize an internal feedback system.27–29
Here we show the fabrication of soft hydrogel objects, namely fibres and beads, that have an individually programmed time delay in their response to a shared environmental stimulus. We utilize the enzyme urease to programme a self-regulated change in pH, which in turn activates the designed response of gel fibre disintegration or a change in gel beads colour.
Remarkably, we not only observe the desired independent response behaviour in a collection of bodies in a closed system, but also inter-material communication on shorter length scales. We believe that the incorporation of response time control and communication into the design of hydrogel objects aids the development of autonomous materials.
Aqueous solutions of sodium alginate (1 wt% for fibres, 5 wt% for beads, at pH 7) were used as the base material to construct hydrogels. Calcium chloride hexahydrate was used as a source for calcium ions to establish ionic cross-linking. Once formed using fluidic devices, these Ca2+ cross-linked hydrogels are stable when dispersed in water over a wide pH range.30
Alginate-based hydrogels are widely used in food, personal care, and pharmaceutical applications due to their biologically friendly nature and ionic cross-linking capabilities, making them an excellent tool for the design of soft materials.31
For fibres, we employed a flow focussing device which allows for coloured oil droplets to be embed along the length. Panels (i) and (ii) of Fig. 1 depict the formation of composite fibres by this method. Oil droplets (red) are formed in a continuous flow of 1 wt% sodium alginate that meets a 0.1 mol dm−3 aqueous solution of calcium chloride hexahydrate, upon which the solution is ionically cross-linked by calcium ions. The oil droplets were used to distinguish fibres from one another as well as a way to track their disassembly. The sodium alginate solution contained varying amounts of the enzyme urease, which together with the oil droplets becomes entrapped upon crosslinking into the hydrogel matrix.32 The enzyme is introduced to indirectly control pH over time within the fibre. It does this by converting urea, added to the liquid environment in which the gels are placed, into ammonia and carbon dioxide. Importantly, the activity of urease is pH dependent, with a bell-shaped activity curve (see panel b material time programming and legend for its mathematical fit, Fig. 1).33
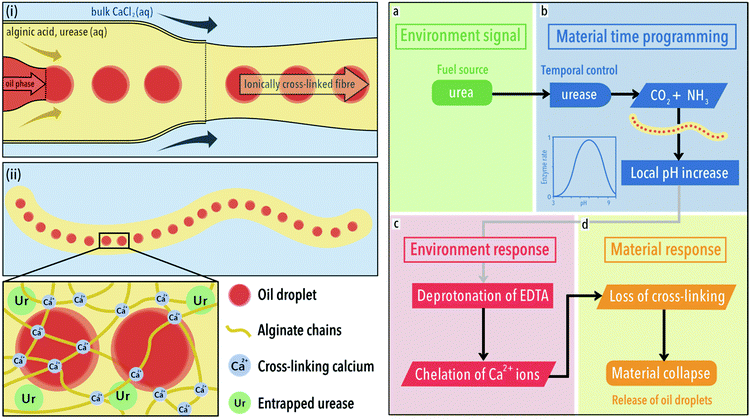 | ||
| Fig. 1 Synthesis and action of composite fibres. Left (i): Formation of calcium ion cross-linked sodium alginate fibres using a microfluidic synthesis. A fluid stream of sodium alginate solution (1 wt%) containing oil droplets meets a bulk solution containing calcium ions (0.1 mol dm−3), upon which an ionically cross-linked gel fibre forms. (ii) The gel network, cross-linked by calcium ions, entraps both the enzyme urease and oil droplets. Right: (a) Following a course of programmed behaviour, the fibre converts urea, the fuel, to ammonia, due to the inclusion of urease in its structure. (b) After a defined time period, the fibre generates a local increase in pH. This time-programming is afforded due to the bell-shaped activity curve of urease, where the relative enzyme rate, v′ = (1 + 2 × 10−9/[H+] + [H+]/5 × 10−6)−1. Graph reproduced from ref. 33. (c) Within the low pH (3.50) bulk environment, partially protonated ethylenediaminetetraacetic acid (EDTA) can be found. As the pH of fibre increases locally, EDTA is deprotonated locally and chelates calcium ions. (d) This results in a loss of cross-linking and the release of oil droplets. | ||
When we place one of our hydrogel fibres (10 g L−1 urease) into acidic water of pH 4 in the presence of urea (0.09 mol dm−3) and bromothymol blue as pH colorimetric indicator, it becomes evident that the local pH rises from within the fibre as a direct result of enzyme activity (see Movie 1, ESI,† and Fig. 2).
Our first goal in autonomous hydrogel material design is to demonstrate programmed time delay in the disintegration of the fibres and thus release of the oil droplets.
The sodium salt of ethylenediaminetetraacetic acid (EDTA) is added to the bulk aqueous solution as a chelating agent for calcium ions, acting as a dormant Ca2+-sink. The initial pH of the aqueous medium containing both EDTA and urea in which the fibres are placed is set at a value of 3.50. EDTA has a maximum binding affinity for Ca2+ at high pH, when all 6 of its donor groups (4 carboxylic acid groups and 2 nitrogens) are ionized. At pH 7.5, only a fraction of the EDTA will be in this form, thus its affinity will be reduced.34 This affinity continues to decrease as the pH is lowered. This was verified by placing a hydrogel fibre into an EDTA solution of pH 3.50 without urea. Without the ability to raise its pH, it remained intact over a period of at least 5 days. Without EDTA, the ionically cross-linked alginate gels are stable for months.
When urea is present in the hydrogel fibre environment, the specific amount of enzyme entrapped within the fibre controls the increase in pH as a function of time (material time programming). The bell-shaped pH-activity curve of urease allows us to use its auto-catalytic behaviour as a tool for time-programming. With low enzyme activity at low pH values the generation of base provides a positive feedback mechanism that elevates the pH into the higher activity window. The transition from low to high pH follows sigmoidal behaviour, which allows for easy introduction of a dormancy period by variation of the enzyme concentration. A higher enzyme concentration results in a shorter dormancy period.
Upon a pH increase inside and in close proximity of the fibre, EDTA transitions to its Ca2+ chelating form, hereby promoting cation exchange. This results in the eventual disintegration of the alginate-gel matrix and subsequent release of the entrapped oil droplets.
To summarise the right-hand panel of Fig. 1, the fibre converts urea, the fuel, to ammonia, due to the inclusion of urease in its structure (the environmental signal, a). After a defined time period, dependent on the concentration of urease, the fibre generates a local increase in pH (material time programming, b). Within the low pH (3.50) bulk environment, partially protonated ethylenediaminetetraacetic acid (EDTA) can be found. As the pH of fibre increases locally, EDTA is deprotonated locally and chelates calcium ions (environmental response, c). The chelation of calcium ions from the gel structure results in a loss of cross-linking and the release of oil droplets (material response, d).
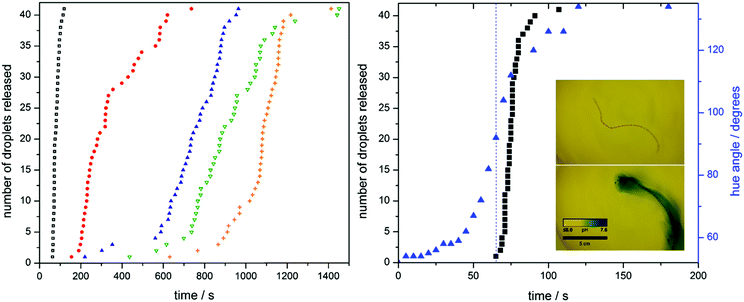
A series of hydrogel fibres each containing 41 oil droplets were fabricated with urease concentrations of 4.0, 5.0, 6.0, 8.0, and 10.0 g L−1. The rate of fibre disintegration was tracked by measuring the release of the entrapped oil droplets (see Fig. 2 and extended Fig. 1). An increase in enzyme loading leads to a shorter dormancy period before hydrogel matrix collapse, characterized by droplet release. To confirm the relationship between droplet release and pH increase, we analysed the colour changes seen in the previously mentioned bromothymol blue indicator experiment (Fig. 2). We quantify this change in colour by measuring the hue angle of the corresponding HSV histogram. The fibre starts with a hue angle of ca. 50°, corresponding to a yellow colour, and finishes with angle of ca. 140°, typical of a blue colour. Not only does the shape of the release curves resemble that of the pH change, but the pH at which fibre disassembly starts can be deduced by means of a hue angle-pH calibration, shown in extended Fig. 2. (Note: the hue is the angle around the central vertical axis of an HSL cylindrical coordinate representation of a colour. In this context, we use it as a means to quantify colour. As the colour of the bromothymol blue indicator changes with pH, we can assign hue values across the pH range by means of a calibration. This allows us to deduce the pH of the fibre from its colour.) Disassembly starts at a pH of approximately 6.3.
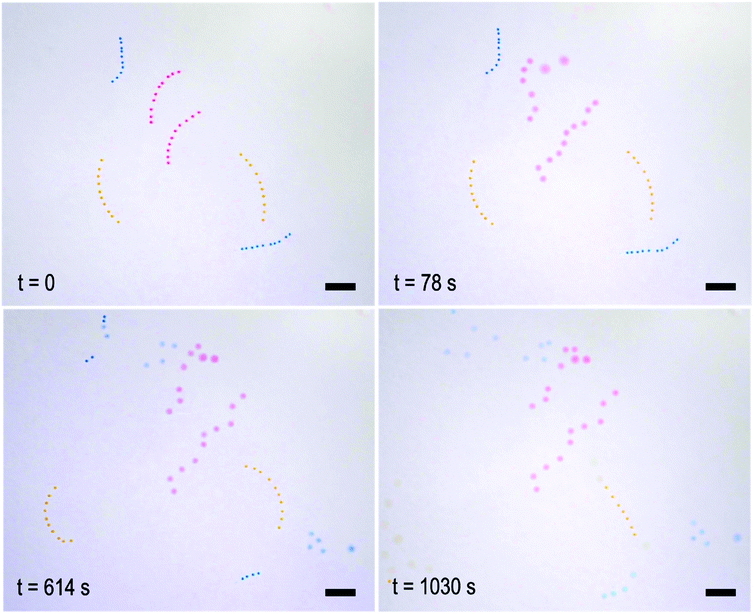
As we can program bespoke time delay in the disintegration behaviour of the fibres, a collection of them can act independently in a single closed system. To demonstrate this, a set of fibres with urease concentrations of 10.0, 8.0 and 4.0 g L−1 were prepared, containing red, blue, and orange oil droplets, respectively. As in the case of the single fibre experiments, a reduction in urease concentration leads to a longer inhibition period, such that the fibres disintegrate in a pre-defined order, as shown in Fig. 3 and Movie 2 (ESI†). The corresponding dormancy time periods were in approximate agreement with the results presented in Fig. 2.
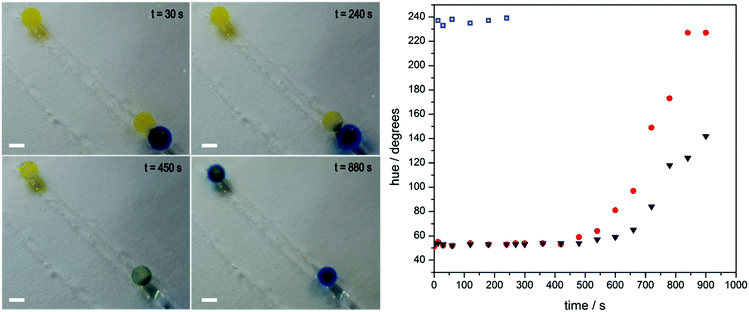
At first glance, self-regulating behaviour means that disintegration of one fibre should not affect the others. Upon closer inspection of the video footage, however, it appears that the disintegration of the red fibre at the top influences that of its less active neighbour, a blue one. This observation prompted us to look at how the gels might communicate with one another, and in turn, the effect this has on their programmed behaviour. For this we designed an experiment (lasting approximately 1000 seconds, see Fig. 4) in which a gel bead of high enzyme concentration (10.0 g L−1) already at high pH, as indicated by its blue colour originating from bromothymol blue, makes temporary contact (240 seconds) with one of two yellow beads of lower urease concentration (0.008 g L−1) in a pH 3.50 urea solution. Contact with the blue bead “infects” the yellow bead and it transitions faster to blue (exceeding a pH of 7.6 as indicated by the presence of bromothymol blue) than its unaffected counterpart. A plausible explanation is that the transient pH increase generated by the first bead drives the lower concentration bead up its bell-shaped pH activity curve due to the diffusion of locally generated base. This push decreases the pre-programmed dormancy period and allows the infected gel bead to reach a higher pH in a shorter time period. We quantify this change in colour as before by measuring the hue angle. The two 0.008 g L−1 beads start with a hue angle of ca. 50°, corresponding to a yellow colour. The 10 g L−1 bead has an angle of ca. 230°, typical of a deep blue colour. Over the course of the experiment the triggered bead reaches this maximum hue angle whilst the un-triggered bead does not. This is due to an increased dormancy period and a slower rate of change.
Conclusions
To conclude, our studies show that it is possible to design and fabricate hydrogel objects that can react with time control in response to a trigger originating from their environment. This provides a level of autonomy and ultimately shifts control from the environment to the material itself. Additionally, communication between nearby hydrogel objects was demonstrated. We believe that the combination of uniquely programmed response behaviour and communication opens up exciting opportunities in the design of soft robotic materials that are capable of signal transduction. As an example, soft robotic objects have to rely on the ability to send signals and act upon them often in complex environments. A chemical signalling pathway is an obvious choice. A collection of synthetic ‘tissues’ or ‘organs’ may receive a shared stimulus, and need to act differently and independently. The ability to build soft systems with independent function operating in the same physical space brings us closer to this biologically inspired design.References
- J. A. Loeser, The Concept of Instinct, Nature, 1939, 143, 880–883 CrossRef.
- E. Bonabeau, M. Dorigo and G. Theraulaz, Inspiration for optimization from social insect behaviour, Nature, 2000, 406, 39–42 CrossRef CAS PubMed.
- J. B. Pollack and H. Lipson, Automatic design and manufacture of robotic lifeforms, Nature, 2000, 406, 974–978 CrossRef PubMed.
- M. Heisenberg, Is free will an illusion?, Nature, 2009, 459, 164–165 CrossRef CAS PubMed.
- R. O. Doyle, Free will: it's a normal biological property, not a gift or a mystery, Nature, 2009, 459, 1052 CrossRef CAS PubMed.
- P. Fratzl and F. G. Barth, Biomaterial systems for mechanosensing and actuation, Nature, 2009, 462, 442–448 CrossRef CAS PubMed.
- D. Rus and M. T. Tolley, Design, fabrication and control of soft robots, Nature, 2015, 521, 467–475 CrossRef CAS PubMed.
- A. R. Morgan, et al., Chemotaxis of catalytic silica–manganese oxide ‘matchstick’ particles, Mater. Horiz., 2014, 1, 65–68 RSC.
- K. K. Dey, et al., Micromotors Powered by Enzyme Catalysis, Nano Lett., 2015, 15, 8311–8315 CrossRef CAS PubMed.
- O. E. Shklyaev, H. Shum, A. Sen and A. C. Balazs, Harnessing surface-bound enzymatic reactions to organize microcapsules in solution, Sci. Adv., 2016, 2, e1501835 Search PubMed.
- C. Miesch and T. Emrick, Photo-sensitive ligands on nanoparticles for achieving triggered emulsion inversion, J. Colloid Interface Sci., 2014, 425, 152–158 CrossRef CAS PubMed.
- R. W. Jaggers, et al., Control of vesicle membrane permeability with catalytic particles, Mater. Horiz., 2016, 3, 41–46 RSC.
- F. Ahmed and D. E. Discher, Self-porating polymersomes of PEG-PLA and PEG-PCL: hydrolysis-triggered controlled release vesicles, J. Controlled Release, 2004, 96, 37–53 CrossRef CAS PubMed.
- S. Yu, T. Azzam, I. Rouiller and A. Eisenberg, ‘Breathing’ Vesicles, J. Am. Chem. Soc., 2009, 131, 10557–10566 CrossRef CAS PubMed.
- K. T. Kim, J. J. L. M. Cornelissen, R. J. M. Nolte and J. C. M. van Hest, A Polymersome Nanoreactor with Controllable Permeability Induced by Stimuli-Responsive Block Copolymers, Adv. Mater., 2009, 21, 2787–2791 CrossRef CAS.
- M. A. C. Stuart, et al., Emerging applications of stimuli-responsive polymer materials, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed.
- E. D’Elia, et al., Autonomous self-healing structural composites with bio-inspired design, Sci. Rep., 2016, 6, 25059 CrossRef PubMed.
- T. Sun, Functional biointerface materials inspired from nature, Chem. Soc. Rev., 2011, 40, 2909 RSC.
- R. Yoshida, Self-oscillating gels driven by the Belousov-Zhabotinsky reaction as novel smart materials, Adv. Mater., 2010, 22, 3463–3483 CrossRef CAS PubMed.
- O. Kuksenok, et al., Chemo-responsive, self-oscillating gels that undergo biomimetic communication, Chem. Soc. Rev., 2013, 42, 7257 RSC.
- S. Ogi, K. Sugiyasu, S. Manna, S. Samitsu and M. Takeuchi, Living supramolecular polymerization realized through a biomimetic approach, Nat. Chem., 2014, 6, 188–195 CrossRef CAS PubMed.
- S. Ogi, T. Fukui, M. L. Jue, M. Takeuchi and K. Sugiyasu, Kinetic Control over Pathway Complexity in Supramolecular Polymerization through Modulating the Energy Landscape by Rational Molecular Design, Angew. Chem., Int. Ed., 2014, 53, 14363–14367 CrossRef CAS PubMed.
- E. Mattia and S. Otto, Supramolecular systems chemistry, Nat. Nanotechnol., 2015, 10, 111–119 CrossRef CAS PubMed.
- A. E. Engelhart, K. P. Adamala and J. W. Szostak, A simple physical mechanism enables homeostasis in primitive cells, Nat. Chem., 2016, 8, 448–453 CrossRef CAS PubMed.
- H. Ye, M. Daoud-El Baba, R.-W. Peng and M. Fussenegger, A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice, Science, 2011, 332, 1565–1568 CrossRef CAS PubMed.
- X. He, et al., Synthetic homeostatic materials with chemo-mechano-chemical self-regulation, Nature, 2012, 487, 214–218 CrossRef CAS PubMed.
- S. Debnath, S. Roy and R. V. Ulijn, Peptide Nanofibers with Dynamic Instability through Nonequilibrium Biocatalytic Assembly, J. Am. Chem. Soc., 2013, 135, 16789–16792 CrossRef CAS PubMed.
- T. Heuser, E. Weyandt and A. Walther, Biocatalytic Feedback-Driven Temporal Programming of Self-Regulating Peptide Hydrogels, Angew. Chem., Int. Ed. Engl., 2015, 54, 13258–13262 CrossRef CAS PubMed.
- T. Heuser, A.-K. Steppert, C. Molano Lopez, B. Zhu and A. Walther, Generic Concept to Program the Time Domain of Self-Assemblies with a Self-Regulation Mechanism, Nano Lett., 2015, 15, 2213–2219 CrossRef CAS PubMed.
- K. Y. Lee and D. J. Mooney, Alginate: properties and biomedical applications, Prog. Polym. Sci., 2012, 37, 106–126 CrossRef CAS PubMed.
- K. Y. Lee and D. J. Mooney, Alginate: properties and biomedical applications, Prog. Polym. Sci., 2012, 37, 106–126 CrossRef CAS PubMed.
- F. Muzika, et al., A bistable switch in pH in urease-loaded alginate beads, Chem. Commun., 2014, 50, 11107 RSC.
- G. Hu, J. A. Pojman, S. K. Scott, M. M. Wrobel and A. F. Taylor, Base-Catalyzed Feedback in the Urea−Urease Reaction, J. Phys. Chem. B, 2010, 114, 14059–14063 CrossRef CAS PubMed.
- Y. V. Griko, Energetics of Ca2+–EDTA interactions: calorimetric study, Biophys. Chem., 1999, 79, 117–127 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods, two extended figures, and three videos. See DOI: 10.1039/c7mh00033b |
| This journal is © The Royal Society of Chemistry 2017 |