Controlling the exciton lifetime of blue thermally activated delayed fluorescence emitters using a heteroatom-containing pyridoindole donor moiety†
Abstract
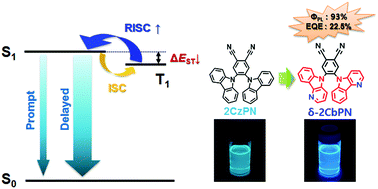
In this paper, we report a promising molecular design concept to attain short exciton lifetime, appropriate triplet energy and high photoluminescence quantum yield (PLQY) in blue thermally activated delayed fluorescence (TADF) emitters for high quantum efficiency and better efficiency roll-off characteristics in organic light emitting diodes (OLEDs). Herein, new TADF molecules with nitrogen at α- and δ-positions of the carboline donor moiety were synthesized and their effects on the photo-physical and electroluminescence properties were theoretically and experimentally investigated. The TADF molecule with a δ-carboline donor moiety was much favorable for short exciton lifetime, high delayed PLQY, good blue color, and small singlet and triplet splitting compared to the generally used carbazole. A maximum external quantum efficiency (EQE) of 22.5%, reduced efficiency roll-off and good color purity with Commission Internationale de l'Eclairage (CIE) 1931 color coordinates of (0.19, 0.34) were exhibited by the blue OLED using the TADF emitter, 4,5-bis(5H-pyrido[3,2-b]indol-5-yl)phthalonitrile (δ-2CbPN). Moreover, the effectiveness of the δ-carboline donor moiety was verified in another deep blue TADF emitter, 5,5′-(2-(9H-carbazol-9-yl)-5-(4,6-diphenyl-1,3,5-triazin-2-yl)-1,3-phenylene)bis(5H-pyrido[3,2-b]indole) (CzDCbTrz). A maximum EQE of 23.4%, deep blue color, and reduced efficiency roll-off were attained in the CzDCbTrz based OLED.
- This article is part of the themed collection: Materials Horizons 10th anniversary regional spotlight collection: Asia-Pacific


 Please wait while we load your content...
Please wait while we load your content...