Open Access Article
Open Access ArticleBreaking the paradigm: record quindecim† charged magnetic ionic liquids‡
D.
Prodius
 a,
V.
Smetana
a,
S.
Steinberg§
a,
M.
Wilk-Kozubek¶
ab,
Y.
Mudryk
a,
V. K.
Pecharsky
ab and
A.-V.
Mudring
a,
V.
Smetana
a,
S.
Steinberg§
a,
M.
Wilk-Kozubek¶
ab,
Y.
Mudryk
a,
V. K.
Pecharsky
ab and
A.-V.
Mudring
 *ab
*ab
aAmes Laboratory, US Department of Energy and Critical Materials Institute, Ames, Iowa 50011-3020, USA. E-mail: mudring@iastate.edu
bDepartment of Materials Sciences and Engineering, Iowa State University, Ames, Iowa 50011-1096, USA
First published on 8th December 2016
Abstract
A family of bis(trifluoromethanesulfonyl)amide-based ionic liquids of composition [RE5(C2H5-C3H3N2-CH2COO)16(H2O)8](Tf2N)15 (RE = Er, Ho, Tm; C3H3N2 ≡ imidazolium moiety) featuring the cationic, record quindecim {15+} charged pentanuclear rare earth (RE)-containing ion [RE5(C2H5-C3H3N2-CH2COO)16(H2O)8]15+ has been synthesized and characterized. In addition, due to the presence of rare earth ions, these ionic liquids show a response to magnetic fields with the highest effective magnetic moment observed so far for an ionic liquid and are rare examples of ionic liquids showing luminescence in the near-infrared. These ionic liquids also were successfully employed in a three-component synthesis of 2-pyrrolo-3′-yloxindole with an extremely low (<0.035 mol%) catalyst loading rate.
Conceptual insightsIonic liquids (ILs) are salts that are liquid at low temperature (<100 °C) which represent a class of new ‘green’ materials with nonmolecular character and a wide range of practical applications. A boundless variety of cation/anion combinations (∼1019) gives us a choice to create an IL for a desired process, device or product. Nevertheless, one of the design limiting criteria and paradigms in IL research is that a combination of highly charged ions will lead to a compound being solid at room temperature (or below 100 °C) because of the high Coulombic attraction. In this communication, we demonstrate that the paradigm of using only low charged ions for creating new ionic liquids should be revisited. These materials not only feature the highest charge ever observed for an ionic liquid, but they are also magnetic ionic liquids with the highest magnetic moment observed ever. Other achievements of this work are the realization of rather challenging NIR luminescence in the liquid state of materials and highly efficient catalytic abilities. |
In the quest for safer and greener technologies, ionic liquids (ILs), commonly defined as salts with a melting point below 100 °C, offer promising technology solutions because of their unique properties and property combinations which can be realized due to their wide tuneability and modular character.1 Not only can they be considered as excellent ‘green’ alternatives to volatile organic solvents (volatile organic compounds, VOCs) because of their generally extremely low vapour pressure, but ILs can also offer useful material properties and unique property combinations such as wide electrochemical window, excellent thermal stability, miscibility, low combustibility, and high catalytic and biologic activity which renders them interesting for a number of special applications. Activities in this field are documented by a large number of scientific publications and patents, the total number doubling over the last five years.2 The number of known IL forming cations and anions lead to 1019 possible combinations resulting in ILs – each with an individual set of chemical and physico-chemical properties – which underpins their remarkable potential.3
One of the design criteria and paradigms in IL research is that the ions constituting the IL have to be of low charge, so that the Coulombic attraction is low, resulting in a reduced thermodynamic stability of the solid.4 Indeed, the majority of identified ILs is composed of singly charged organic quaternary cations and inorganic anions.4 So far, the highest charge (+7) of an ion in ionic liquid was observed for the μ3-oxo cluster cation, [Fe3O(cmmim)6(H2O)3]7+ (cmmim = 1-carboxymethyl-3-methylimidazolium), in [Fe3O(cmmim)6(H2O)3](Tf2N)7 (Tf2N = bis(trifluoromethanesulfonyl)amide, F3C–SO2–N−–SO2–CF3 with a melting point of Tm = 92 °C).5 Incorporation of metals into ionic liquids offer the potential to bestow the ionic liquid with additional chemical and physical properties.6 In this light, rare earth cations are well-suited to endow a range of ionic liquids with additional features, as rare earth compounds are well known for their outstanding luminescence, magnetism and catalytic activity.7 After the first report on a rare earth containing ionic liquid with divalent rare earth cations,8 seminal publications on rare earth containing ionic liquids with luminescent9 and magnetic properties10 have stimulated the field and a fair number of ionic liquids with complex rare earth containing cations11 and anions9,10,12 have been reported over the last few years. In this communication, we report on the synthesis and characterization of a new family of rare earth-containing liquids, [RE5(C2H5-C3H3N2-CH2COO)16(H2O)8](Tf2N)15 (RE = Er, Ho, Tm; C2H5-C3H3N2-CH2COO ≡ 1-carboxymethyl-3-ethylimidazolium), featuring a novel pentanuclear rare earth cation with a charge of +15. This type of cation represents the highest charged ion for an ionic liquid achieved so far.
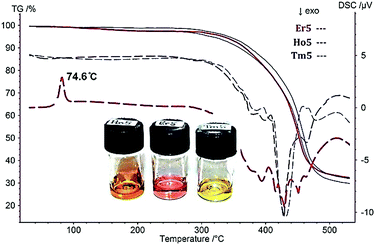
The [RE5(C2H5-C3H3N2-CH2COO)16(H2O)8](Tf2N)15 (1–3) compounds can be obtained by reacting the respective rare oxide with 1-carboxymethyl-3-ethylimidazolium chloride and LiTf2N in aqueous solution (for details see the ESI‡) as liquids forming a separate phase (Fig. 1, inset). The ILs overall show a low tendency to crystallize. For the Er (1) and Ho (2) ILs, crystals form after a few months under ambient conditions.
The thermal behaviour of all compounds was investigated by differential scanning calorimetry (DSC) and thermogravimetry (TG) (Fig. 1). Crystalline [Er5(C2H5-C3H3N2-CH2COO)16(H2O)8](Tf2N)15 shows a melting point of 74.6 °C, thus truly qualifying as an IL. Upon cooling, 1 vitrifies at 10 °C (at a thermal ramp of 10 °C min−1) and partial crystallization occurs at −32 °C. Upon subsequent heating the IL devitrifies at 0 °C. In the following thermal cycles all glass transitions have been observed, however, without crystallization. A similar thermal behaviour has been detected for the Ho IL (2). All crystallization attempts for Tm IL (3) failed and only glass transitions were observed (for details on the thermal behaviour see the ESI‡). This illustrates the extremely low tendency of these compounds to crystallize and all ILs are metastable RTILs (room temperature ILs). The thermal stability of 1–3 was investigated by TG. Dehydration occurring around at 110 °C was identified as a first decomposition step. The observed weight loss of 2.35% at ∼200 °C corresponds to the elimination of all eight water molecules.
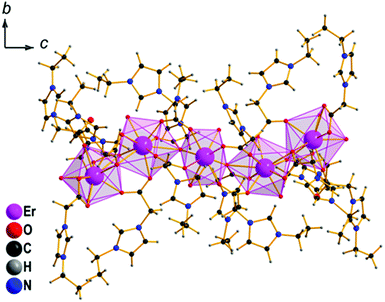
To identify the structural units in these complex ionic liquids, single crystal X-ray structure analysis was applied (Fig. 2). It has to be stressed that the growth of crystals of sufficient quality for X-ray structure analysis is extremely difficult due to the low tendency of the compounds to crystallize. In addition, structure solution and refinement is not trivial due to the large number of independent parameters that need to be refined. The Er- and Ho- compounds are isotypic and crystallize in the non-centrosymmetric orthorhombic space group Pca21 (ESI,‡ Table S1) and feature the quindecim charged [Er5(C2H5-C3H3N2-CH2COO)16(H2O)8]15+ unit which is composed of five crystallographically independent Er atoms (Fig. 2). The structure of the Er compound will be discussed in detail as a representative of the series.
The central Er3+ cation is octacoordinated in the form of a distorted square-antiprism, while the other four feature 9-fold coordination spheres formed by oxygen atoms. The latter coordination polyhedra can be best described as axially capped square antiprisms with different degrees of distortion (ESI,‡ Fig. S2–S4). Slight, but distinct differences in the Er coordination spheres destroy the center of inversion for the complex polycation. Refinement in the corresponding centrosymmetric space group Pcam fails.
The central ErO8 polyhedron shares two vertices with the second and fourth, which, in turn, share edges with the peripheral units. The observed Er–O distances range from 2.241(9) to 2.553(8) Å. These values lie in the typical range for Er(III) carboxylates.13 The central Er3+ cation with a lower coordination number shows, as expected, shorter Er–O distances than the others with a higher coordination number (2.25(1)–2.42(1) Å), while a small fraction of the Er–O contacts can reach even values of 2.7–2.82 Å as a result of distortion due to higher coordination (η2:η1:μ2 mode) and bridging between two Er atoms. Tf2N anions surrounding the complex {Er5} compensate the charge of the cation. They exhibit both cisoid- and transoid-conformation with respect to the S–N–S bond with a significant domination (11 vs. 4) of the latter. The transoid conformation is the thermodynamically favoured one in the gas phase and is also the more stable in the solid if no specific interactions stabilize the cisoid.8
The IR spectra of 1 in the solid and liquid state are similar, indicating that the same structural units are present in both in the liquid and the solid state, most importantly the [Er5(C2H5-C3H3N2-CH2COO)16(H2O)8]15+ cation preserves structural integrity (ESI‡). As expected, the IR spectra of the analogous compounds 2 and 3 feature the same characteristic vibrations as 1 (ESI‡).
Electrospray mass spectra of all aqueous solutions of 1–3 widely show matching peaks, confirming not only their structural identity, but most importantly proof the existence of the pentanuclear rare earth cation core, even in solution (Fig. S8 and Table S3, ESI‡).
The magnetic properties of 1–3 were measured in the temperature range from 2 to 300 K and in an applied magnetic field of 1000 Oe (Fig. 3 and Tables S4, S5 and Fig. S9, S10 in the ESI‡).
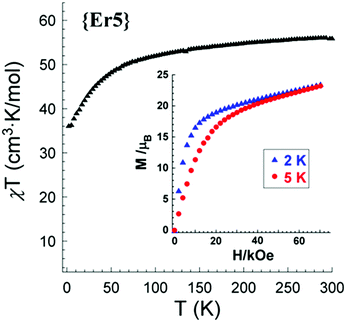 | ||
| Fig. 3 Plot of χMT vs. T for compound 1 under 1000 Oe dc field and molar magnetization (M) vs. applied field (H) at 2 and 5 K (inset). | ||
The magnetization (M) data were collected in the 0–70 kOe field range at different temperatures (2 and 5 K). The room-temperature χT values of complexes 1, 2, and 3 are 56.2, 67.9, and 32.9 cm3 K mol−1, respectively. These results are in good agreement with the theoretical values expected for five noninteracting rare earth ions (1, 57.4 cm3 K mol−1; 2, 70.35 cm3 K mol−1; 3, 35.75 cm3 K mol−1) according to the single ion values at 300 K.13b Upon lowering the temperature, χT for 1–3 remains almost constant until ∼150 K and then decreases sharply to reach a value of 36.2, 31.5, and 14.7 cm3 K mol−1 at 2.0 K for compounds 1 (Fig. 3), 2 and 3, respectively (Fig. S9, ESI‡).
Using room temperature χT values, we calculated effective paramagnetic moments in the liquid state for 1–3 (see the ESI,‡ Table S4), which are equal to 21.2 (1), 23.3 (2), and 16.2 (3) μB. Yet, with these effective magnetic moment (μeff) values all three ILs exceed the highest μeff so far reported for an ionic liquid (11.76 μB).14 Thus, all ionic liquids respond to external magnetic fields and belong to the group of magnetic ionic liquids which recently have found application in DNA separation.14b
The field dependence of the magnetization for 1–3 (inset, Fig. 3 and Fig. S9, ESI‡) at low temperatures (2 and 5 K) shows that the magnetization increases rapidly at low field and finally reaches values of 23.2 (1), 28.1 (2), and 15.0 (3) μB at 2 K (H = 70 kOe) without clear saturation. These values are much lower than the expected saturation values of five non-interacting Er3+, Ho3+, and Tm3+ ions. The low values of saturation may originate from magnetic anisotropy and/or the lack of a well-defined ground state.
Erbium(III) and holmium(III) are two ions that are well known to exhibit luminescence in the NIR. Luminescence in this spectral range is of interest for biomedical imaging, telecommunications and solar energy conversion. However, due to the small energy gap between the excited and ground state NIR luminescence is easily quenched by vibronic coupling with O–H, N–H and C–H vibrations from water and organic ligands and hard to observe in the liquid state. For Er3+ compounds only two O–H vibrations need to get activated to allow for a radiationless return to the ground state. No luminescent Ho3+ ILs have been reported so far. We have reported the luminescence of ErI3 in the highly viscous IL [C12mim]Tf2N (C12mim = 1-dodecyl-3-methylimidazolium)15 and Bünzli and co-workers studied the optical behaviour of [RE(tta)3(phen)] (tta = thenoyltrifluoroacetonate, phen = 1,10-phenanthroline) in the ionic liquid crystalline material [C12mim]Cl.16 Near infrared luminescence measurements for 1 and 2 were performed at room temperature (Fig. S12–S17, ESI‡). The NIR emission of 1 in a liquid state is observed when excited at 524 nm (Fig. S13, ESI‡). A broad band at 1540 nm with four shoulders (1480, 1510, 1560 and 1590 nm) can be assigned to the 4I13/2 → 4I15/2 transition of the Er3+ ion. The emission spectrum of 1 in the solid state is similar to that in the liquid state (Fig. S15, ESI‡). The lifetime of 1, in both liquid and solid states, is 0.6 μs. Upon excitation at 487 nm, 2 shows the characteristic NIR luminescence of the Ho3+ ion (Fig. S17, ESI‡). The emission spectrum consists of bands with maxima at 980, 1237 and 1478 nm, which are attributed to 5F5 → 5I7, 5I6 → 5I8 and 5F5 → 5I6 transitions of the Ho3+ ion. The lifetime of 2 is 0.8 μs. The lifetimes are appreciably high for a compound in the liquid state, especially when taking into account that the rare earth cations are surrounded by water molecules and organic ligands were typically lifetimes in the ns regime are found.17 The observed lifetimes are similar to those found for glasses and complexes with rigid ligand surrounding.18 However, they are a little shorter than that observed for ErI3 doped into the highly viscous IL [C12mim]Tf2N (τ = 10.4 μs).16
The ILs synthesized not only show intriguing properties as neat ILs, but also in solution where the quindecim charge cation can serve as an efficient catalyst. Oxindoles as well as pyrroles are among the most prevalent heterocyclic compounds being present as the basic cores in many potent pharmaceutical compounds, natural products and various kinds of useful materials.19 Multicomponent reactions continue to be the center of attention for the preparation of these compounds since three or more molecular building blocks can simultaneously be combined into one target substance.20 As part of outspreading work on the synthesis of hybrids of both heterocyclic moieties of the above mentioned compounds, 1–3 have been tested as catalysts to the known21 synthesis of ethyl 2-methyl-4-(2-oxo-2,3-dihydro-1H-3-indolyl)-5-phenyl-1H-3-pyrrolecarboxylate (5) through three-component coupling of 3-phenacylideneoxindole (4a), ammonium acetate (4b) and ethyl acetoacetate (4c) (Scheme 1 and Fig. S11, ESI‡). About 0.03 mol% of ILs (1–3) catalyzed a one-pot reaction with ca. 96–99% yield and satisfy the general requirements of scale-up catalyst loadings (<0.1 mol%).22 Increasing the molar ratio of 1–3 up to 3 mol% did not improve the yield noticeably. The high purity, good yields and easy separation of 5 by simple filtration, encouraged us to investigate the reuse and recycling of our ionic liquids.
After separation of product 5 by simple decantation, the residue was directly recycled in the subsequent runs. As shown in Table S6 (ESI‡), the catalysts can be reused at least ten times without significant loss of activity (ca. 85–87%).
Conclusions
In conclusion, we have demonstrated the syntheses and structural characterization of the record quindecim charged pentanuclear rare earth based ionic liquids. Mass spectrometry (ESI-MS) in combination with single crystal X-ray analysis has proven to be an exceptionally vital tool for characterizing the metal containing ILs in liquid/solid states. With room temperature μeff (Bohr magnetons) values as high as 21.2 (1), 23.3 (2) and 16.2 (3) the effective moments are the highest ever reported for magnetic ionic liquids (MILs). The ionic liquids can not only respond to a magnetic field, but also show NIR luminescence and extraordinary catalytic activity. They can be used in the three-component synthesis of ethyl 2-methyl-4-(2-oxo-2,3-dihydro-1H-3-indolyl)-5-phenyl-1H-3-pyrrolecarboxylate at extremely low loading levels (0.029–0.031 mol%).Acknowledgements
Financial support from the NSF (CHE-1465071) for the development and synthesis of materials is gratefully acknowledged. Crystallographic studies were supported by the Critical Materials Institute, an Energy Innovation Hub funded by the U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Advanced Manufacturing Office. Magnetic property measurements and crystallographic studies were supported by the Division of Materials Science and Engineering, Basic Energy Sciences Programs, Office of Science of the US Department of Energy under contract No. DE-AC02-07CH11358 with Iowa State University. The authors gratefully acknowledge Dr Oleksandr Dolotko and Dr Tarek Alammar (AmesLab) for their assistance with the powder X-ray diffraction analysis and photoluminescence experiments, and Dr Valeriu Mereacre (KIT, Karlsruhe/Germany) for helpful discussions on the magnetic part of this work.Notes and references
- (a) K. R. Seddon, J. Chem. Technol. Biotechnol., 1997, 68, 351 CrossRef CAS; (b) T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS PubMed; (c) P. Wasserscheid and W. Keim, Angew. Chem., 2000, 112, 3926 ( Angew. Chem., Int. Ed. , 2000 , 39 , 3772 ) CrossRef; (d) M. Antonietti, D. Kuang, B. Smarsly and Y. Zhou, Angew. Chem., Int. Ed., 2004, 43, 4988 CrossRef CAS PubMed; (e) N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123 RSC; (f) H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519 RSC; (g) D. R. MacFarlane, N. Tachikawa, M. Forsyth, J. M. Pringle, P. C. Howlett, G. D. Elliott, J. H. Davis Jr, M. Watanabe, P. Simon and C. A. Angell, Energy Environ. Sci., 2014, 7, 232 RSC.
- SciFinder, Chemical Abstracts Service: ionic liquids, 2016; http://https://scifinder.cas.org.
- (a) J. D. Holbrey and K. R. Seddon, Clean Technol. Environ. Policy, 1999, 1, 223 CrossRef; (b) E. I. Izgorodina, Phys. Chem. Chem. Phys., 2011, 13, 4189 RSC.
- A.-V. Mudring, Aust. J. Chem., 2010, 63(4), 544 CrossRef CAS.
- D. Prodius, F. Macaev, E. Stingaci, V. Pogrebnoi, V. Mereacre, G. Novitchi, G. E. Kostakis, C. E. Anson and A. K. Powell, Chem. Commun., 2013, 49, 1915 RSC.
- (a) S. Hayashi and H. Hamaguchi, Chem. Lett., 2004, 33, 1590 CrossRef CAS; (b) A. V. Mudring and S. Tang, Eur. J. Inorg. Chem., 2010, 2569 CrossRef CAS; (c) Y. Yoshida and G. Saito, Phys. Chem. Chem. Phys., 2010, 12, 1675 RSC; (d) J. Estager, J. D. Holbrey and M. Swadzba-Kwasny, Chem. Soc. Rev., 2014, 43, 847 RSC; (e) J. Klingele, Coord. Chem. Rev., 2015, 292, 15 CrossRef CAS.
- D. A. Atwood, The Rare Earth Elements: Fundamentals and Applications, John Wiley & Sons, Chichester, 2012 Search PubMed.
- A.-V. Mudring, A. Babai, S. Arenz and R. Giernoth, Angew. Chem., 2005, 117, 5621 ( Angew. Chem., Int. Ed. , 2005 , 44 , 5485 ) CrossRef . Once molten, the compound [mppyr]2[Yb(Tf2N)4] shows a high reluctance to crystallize and can be kept liquid at room temperature for months.
- S. Tang, A. Babai and A.-V. Mudring, Angew. Chem., Int. Ed., 2008, 47, 7631 CrossRef CAS PubMed.
- B. Mallick, B. Balke, C. Felser and A.-V. Mudring, Angew. Chem., 2008, 120, 7747 ( Angew. Chem., Int. Ed. , 2008 , 47 , 7635 ) CrossRef.
- (a) K. Binnemans, C. A. Goerller-Walrand, P. Nockemann and B. Thijs, WO 2007147222A2-20071227, 2007; (b) B. Thijs, Task-Specific Ionic Liquids for Solubilizing Metal Compounds, PhD thesis, University of Leuven, 2007 Search PubMed; (c) D. Prodius, F. Macaev, Y. Lan, G. Novitchi, S. Pogrebnoi, E. Stingaci, V. Mereacre, C. E. Anson and A. K. Powell, Chem. Commun., 2013, 49, 9215 RSC.
- (a) A. Babai and A.-V. Mudring, Chem. Mater., 2005, 17, 6230 CrossRef CAS; (b) A. Babai and A.-V. Mudring, Inorg. Chem., 2006, 45, 4874 CrossRef CAS PubMed; (c) A. Babai and A.-V. Mudring, Dalton Trans., 2006, 1828 RSC; (d) A. Babai, Rare-earth complexes in ionic liquids: Structures, electrochemical and optical properties, PhD thesis, University of Cologne, 2006 Search PubMed; (e) P. Nockemann, B. Thijs, N. Postelmans, K. Van Hecke, L. Van Meervelt and K. Binnemans, J. Am. Chem. Soc., 2006, 128, 13658 CrossRef CAS PubMed; (f) A. Babai and A.-V. Mudring, Z. Anorg. Allg. Chem., 2008, 634, 938 CrossRef CAS; (g) R. E. Del Sesto, T. M. McCleskey, A. K. Burrell, G. A. Baker, J. D. Thompson, B. L. Scott, J. S. Wilkes and P. Williams, Chem. Commun., 2008, 447 RSC; (h) G.-H. Tao, Y. Huang, J. A. Boatz and J. M. Shreeve, Chem. – Eur. J., 2008, 14, 11167 CrossRef CAS PubMed; (i) S.-F. Tang and A.-V. Mudring, Eur. J. Inorg. Chem., 2009, 2769 CrossRef CAS; (j) A. Getsis, B. Balke, C. Felser and A.-V. Mudring, Cryst. Growth Des., 2009, 9, 4429 CrossRef CAS; (k) S.-F. Tang, J. Cybinska and A.-V. Mudring, Helv. Chim. Acta, 2009, 92, 2375 CrossRef CAS; (l) A. Getsis and A.-V. Mudring, Eur. J. Inorg. Chem., 2010, 2172 CrossRef CAS; (m) A. S. R. Chesman, M. Yang, B. Mallick, T. M. Ross, I. A. Gass, G. B. Deacon, S. R. Batten and A.-V. Mudring, Chem. Commun., 2012, 48, 124 RSC; (n) A. S. R. Chesman, M. Yang, N. D. Spiccia, G. B. Deacon, S. R. Batten and A.-V. Mudring, Chem. – Eur. J., 2012, 18, 9580 CrossRef CAS PubMed; (o) S.-P. Ji, M. Tang, L. He and G.-H. Tao, Chem. – Eur. J., 2013, 19, 4452 CrossRef CAS PubMed; (p) C. C. L. Pereira, S. Dias, I. Coutinho, J. P. Leal, L. C. Branco and C. A. T. Laia, Inorg. Chem., 2013, 52, 3755 CrossRef CAS PubMed; (q) L. He, S.-P. Ji, N. Tang, Y. Zhao and G.-H. Tao, Dalton Trans., 2015, 44, 2325 RSC; (r) Y. Zhao, L. He, N. Tang, S. Qin, G.-H. Tao and F.-X. Liang, Eur. J. Inorg. Chem., 2015, 542 CrossRef CAS; (s) N. Tang, Y. Zhao, L. He, W.-L. Yuan and G.-H. Tao, Dalton Trans., 2015, 44, 8816 RSC; (t) C. Lu, S. Das, N. Siraj, P. K. S. Magut, M. Li and I. M. Warner, J. Phys. Chem. A, 2015, 119(20), 4780 CrossRef CAS PubMed; (u) J. Alvarez-Vicente, S. Dandil, D. Banerjee, H. Q. N. Gunaratne, S. Gray, S. Felton, G. Srinivasan, A. M. Kaczmarek, R. Van Deun and P. Nockemann, J. Phys. Chem. B, 2016, 120, 5301 CrossRef CAS PubMed.
- (a) C. R. Groom and F. H. Allen, Angew. Chem., Int. Ed., 2014, 53, 662 CrossRef CAS PubMed; (b) CSD version 5.36, 2015, http://www.ccdc.cam.ac.uk/solutions/csd-system/components/csd/; (c) C. Benelli and D. Gatteschi, Introduction to Molecular Magnetism: From Transition Metals to Lanthanides, Wiley-VCH, Weinheim, 2015 Search PubMed.
- (a) O. Nacham, K. D. Clark, H. Yu and J. L. Anderson, Chem. Mater., 2015, 27, 923 CrossRef CAS; (b) K. D. Clark, O. Nacham, J. A. Purslow, S. A. Pierson and J. L. Anderson, Anal. Chim. Acta, 2016, 934, 9 CrossRef CAS PubMed.
- A. Arenz, A. Babai, K. Binnemans, K. Driesen, R. Giernoth, A.-V. Mudring and P. Nockemann, Chem. Phys. Lett., 2005, 402, 75 CrossRef.
- L. Puntus, K. J. Schenk and J.-C. G. Bünzli, Eur. J. Inorg. Chem., 2005, 4739 CrossRef CAS.
- L. Winkless, R. H. C. Tan, Y. Zheng, M. Motevalli, P. B. Wyatt and W. P. Gillin, Appl. Phys. Lett., 2006, 89, 111115 CrossRef.
- S. Comby and J.-C. G. Bünzli, in Handbook on the Physics and Chemistry of Rare Earths, ed. K. A. Gschneidner Jr., J. -C. G. Bünzli and V. P. Pecharsky, North Holland/Elsevier, 2007, vol. 37, p. 217 Search PubMed.
- (a) J. G. Taylor, A. V. Moro and C. R. D. Correia, Eur. J. Org. Chem., 2011, 1403 CrossRef CAS; (b) L. Jiang and Y. C. Chen, Catal. Sci. Technol., 2011, 1, 354 RSC; (c) A. R. Katritzky and S. Rachwal, Chem. Rev., 2010, 110, 1564 CrossRef CAS PubMed; (d) A. V. Lygin and A. Meijere, Angew. Chem., 2010, 122, 9280 ( Angew. Chem., Int. Ed. , 2010 , 49 , 9094 ) CrossRef.
- M. A. P. Martins, C. P. Frizzo, D. N. Moreira, N. Zanatta and H. G. Bonacorso, Chem. Rev., 2008, 108, 2015 CrossRef CAS PubMed.
- G. Shanthi and R. T. Perumal, Tetrahedron Lett., 2009, 50, 3959 CrossRef CAS.
- M. L. Crawley and B. M. Trost, Applications of transition metal catalysis in drug discovery and development: an industrial perspective, Wiley, Hoboken, 2012 Search PubMed.
Footnotes |
| † Quindecim (lat.) – fifteen. |
| ‡ Electronic supplementary information (ESI) available: Detailed experimental procedures, crystallographic information (CIF) for 1, additional characterization data (SXRD, TG, DSC, IR, ESI-MS, magnetic and photoluminescence properties, NMR, catalytic activity) and discussion, including Fig. S1–S17 and Tables S1–S7 (PDF). CCDC 1469873. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6mh00468g |
| § Institute of Inorganic Chemistry, RWTH-Aachen University, Aachen, Germany. |
| ¶ Department of Nanotechnology, Wrocław Research Centre EIT+, 147 Stabłowicka St., 54-066 Wrocław, Poland. |
| This journal is © The Royal Society of Chemistry 2017 |