Evaluation of circularly polarized luminescence in a chiral lanthanide ensemble†
Received
30th August 2017
, Accepted 20th September 2017
First published on 3rd October 2017
Abstract
This work demonstrates a methodology for the evaluation of circularly polarized luminescence of a chiral europium(III) (EuIII) complex species in an ensemble system. The chiral EuIII complex species consists of chiral bis(oxazolinyl)pyridine [(R)- or (S)-iPr-Pybox] and β-diketonate ligands with a pendant nitro group (DK-NO2). The pendant NO2 group in [iPr-Pybox](EuIII)(DK-NO2)3 (monometallic EuIII species) coordinates to the EuIII center of another EuIII complex, giving rise to the generation of a chiral EuIII ensemble consisting of mono-, di-, and the other oligomeric EuIII species, {[iPr-Pybox](EuIII)(DK-NO2)3}n. The luminescence dissymmetry factors (glum) of the chiral EuIII ensemble have been successfully determined by using a commercially available fluorescence spectrophotometer attached with a rotatable λ/4 filter and a fixed linearly polarized plate. This study suggests that the chiral EuIII ensemble in solution displays a large circularly polarized luminescence (|glum| = 0.19) as compared to that of a reference monometallic complex [iPr-Pybox](EuIII)(DK-CN)3 (|glum| = 0.11–13). The larger glum is primarily attributed to the contribution of the dimetallic EuIII species exhibiting a high degree of circular polarization in luminescence (glum2 = 0.27) in the ensemble. Conversely, a large linearly polarized component was observed in luminescence from the chiral EuIII ensemble in the solid state (KBr pellet).
Design, System, Application
This work demonstrated the quantitative evaluation of circularly polarized luminescence in a chiral europium(III) (EuIII) ensemble consisting of several EuIII complex species. Circularly polarized luminescence (CPL) has become one of the hottest research topics in photochemistry due to its intriguing photonic applications, including circularly polarized electroluminescence (CPEL) for 3D displays, quantum information processing and optical data storage. Typically, chiral lanthanide complexes are promising circularly polarized luminescence materials in view of obtaining a high degree of circular polarization in luminescence because of their remarkable emission features of magnetically-allowed intraconfigurational f–f transitions. In this context, the recent rapid development of supramolecular lanthanide chemistry has provided us new opportunities for the evaluation of circularly polarized luminescence in an ensemble consisting of several chiral lanthanide complex species, where each complex exhibits different chiroptical properties. This work describes how to evaluate the luminescence dissymmetry factor of the chiral EuIII ensemble. The present work would expand the scope of the design of molecules and systems with intriguing chiroptical properties.
|
Introduction
Circularly polarized luminescence (CPL) has become one of the hottest research topics in photochemistry. The main reason may be due to its intriguing photonic applications, including circularly polarized electroluminescence (CPEL) for 3D displays, quantum information processing and optical data storage.1–3 Typically, chiral lanthanide complexes are promising circularly polarized luminescence materials in view of obtaining a high degree of circular polarization in luminescence because of their remarkable emission features of magnetically-allowed intraconfigurational f–f transitions.4–11 The magnetic dipole transition satisfies the magnetic-dipole selection rule, ΔJ = 0, ± 1 (except 0 ↔ 0), thus often exhibiting extremely large circular polarization in luminescence.12,13 In this context, the recent rapid development of supramolecular lanthanide chemistry14–18 has provided us new opportunities for the evaluation of circularly polarized luminescence in an ensemble consisting of several chiral lanthanide complex species, where each complex exhibits different chiroptical properties (e.g., luminescence dissymmetry factor). Thus, a simple evaluation methodology for circularly polarized luminescence of a chiral lanthanide ensemble would expand the scope of supramolecular chiral lanthanide systems.
In the present work, we demonstrate the evaluation of circularly polarized luminescence in a chiral europium(III) (EuIII) ensemble consisting of several EuIII complex species (Fig. 1).19 The chiral EuIII complex species consists of chiral bis(oxazolinyl) pyridine [(R)- or (S)-iPr-Pybox] and β-diketonate ligands acting as antennas for generating EuIII luminescence. The β-diketonate ligand (DK-NO2) contains a pendant nitro group which serves as a second coordination site for the EuIII center.20 The chiral EuIII complex ([(R)- or (S)-iPr-Pybox](EuIII)(DK-NO2)3) was employed as a simple cross-linked complex to afford the chiral lanthanide ensemble systems. The monometallic EuIII species spontaneously self-associates in solution conditions, giving rise to the generation of a chiral EuIII ensemble consisting of mono-, di-, and the other oligomeric EuIII species, {[(R)- or (S)-iPr-Pybox](EuIII)(DK-NO2)3}n. We have chosen the ligand pair (iPr-Pybox and DK-NO2) based on our previous work, where tris[β-diketonate (1,1,1,5,5,5-hexafluoropentane-2,4-dione)] EuIII complexes containing the chiral iPr-Pybox ligand exhibited strong circularly polarized luminescence with good emission efficiency.21 The present work revealed that the chiral EuIII ensemble displays a strong circularly polarized luminescence (|glum| = 0.19) mainly due to the contribution of the dimetallic chiral EuIII species with a large luminescence dissymmetry factor (|glum2| = 0.27) in the chiral ensemble.
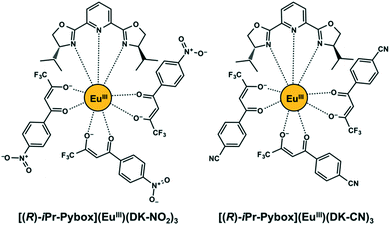 |
| | Fig. 1 Chemical structures of [(R)-iPr-Pybox](EuIII)(DK-NO2)3 and [(R)-iPr-Pybox](EuIII)(DK-CN)3. | |
Experimental
General
Chemicals were purchased from Wako Pure Chemical Industries Ltd. and used as received without further purification. (R,R)- and (S,S)-2,6-bis(4-isopropyl-2-oxazolin-2-yl)pyridine were obtained from Tokyo Chemical Industry Co., Ltd. (TCI). 4,4,4-Trifluoro-1-(4-nitrophenyl)butane-1,3-dione and 4,4,4-trifluoro-1-(4-cyanophenyl)butane-1,3-dione (DK-NO2 and DK-CN, respectively) were prepared according to the procedure described previously.22 The emission lifetimes were recorded using a FluoroCube (HORIBA, 3000 U-YSP). The positive ESI mass spectra of the chiral EuIII complexes were measured with mass spectrometers (JEOL AccuTOF CS JMS-T100CS for ESI). The emission and UV-vis absorption spectra were measured at room temperature using JASCO FP-6500 and V-660, respectively.
Synthesis of [iPr-Pybox](EuIII)(DK-NO2)3 and [iPr-Pybox](EuIII)(DK-CN)3
Precursor complexes, [(EuIII)(DK-NO2)3(H2O)2] and [(EuIII)(DK-CN)3(H2O)2], were prepared as described in the literature.23 Typically, [(R)-iPr-Pybox](EuIII)(DK-NO2)3 was synthesized as follows. (R,R)-2,6-bis(4-isopropyl-2-oxazolin-2-yl)pyridine (0.17 mmol) and [(EuIII)(DK-NO2)3(H2O)2] (0.17 mmol) were dissolved in methanol (30 mL) in a reaction flask. The reaction mixture was stirred overnight at room temperature. After removing the solvent by evaporation, the obtained white powder was washed with distilled water and dried under vacuum (yield: 73%). HRMS [ESI-MS (positive)]: the monometallic complex, m/z calcd. for C47H38EuF9N6O14Na+ [[iPr-Pybox](EuIII)(DK-NO2)3 + Na]+, 1257.14122; found 1257.14024, the dimetallic complex: m/z calcd. for C94H76Eu2F18N12O28Na+ [{[iPr-Pybox](EuIII)(DK-NO2)3}2 + Na]+ 2491.30147, found 2491.29268. [(S)-iPr-Pybox](EuIII)(DK-NO2)3 and reference chiral EuIII complexes, [(R)- or (S)-iPr-Pybox](EuIII)(DK-CN)3 were prepared using the same procedures as those for [(R)-iPr-Pybox](EuIII)(DK-NO2)3. HRMS [ESI-MS (positive)]: m/z calcd. for C50H38EuF9N6O8Na+ [[iPr-Pybox](EuIII)(DK-CN)3 + Na]+ 1197.17174, found 1197.16884.
Determination of the luminescence dissymmetry factor
The luminescence dissymmetry factor (glum) of the chiral EuIII complexes was determined using the experimental setup designed based on a fluorescence spectrofluorometer (JASCO FP-6500) with a rotatable λ/4 filter and a fixed linearly polarized plate (Fig. 5).14a The emission was corrected after passing through the rotatable λ/4 filter and the fixed linearly polarized plate every two degrees of the angle (θ) between the rotatable λ/4 filter and the fixed linearly polarized plate. The observed emission intensity (Iobs) periodically decreases and increases in response to the angle (θ) [Fig. 6a and b], wherein the intensity at the angle θ = 45, 225 and that at 135, 315 degrees correspond to the right- and left-CPL intensity, respectively (IR and IL, respectively) from the sample. The glum values were calculated by using the equation, glum = 2(IL − IR)/(IL + IR). The system precision is checked by using a standard material, a cesium tetrakis(3-heptafluoro-butylryl-(+)-camphorato) EuIII complex, Cs+[Eu((+)-hfbc)4]−. The determined glum value (1.41) is close to the literature value (glum = 1.38).13 These details are further outlined in the text (vide infra).
Results and discussion
Chiral EuIII ensemble
Chiral EuIII complexes with the general formula [(R)- or (S)-iPr-Pybox](EuIII)(DK-NO2)3 were synthesized by reacting the tris(DK-NO2) EuIII complex [(EuIII)(DK-NO2)3(H2O)2] with (R)- or (S)-iPr-Pybox in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry in methanol. Interestingly, the resulting chiral EuIII complex displays a non-single-exponential emission decay in acetonitrile solution (Fig. 2a, λem = 618 nm due to the 5D0 → 7F2 transition of EuIII).14 The decay curve can be fitted as a sum of three exponential decay components [I(t) = A1
1 stoichiometry in methanol. Interestingly, the resulting chiral EuIII complex displays a non-single-exponential emission decay in acetonitrile solution (Fig. 2a, λem = 618 nm due to the 5D0 → 7F2 transition of EuIII).14 The decay curve can be fitted as a sum of three exponential decay components [I(t) = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2
exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) + A3
exp(−t/τ2) + A3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ3)] with τ1 = 0.26 ms (11.2%), τ2 = 0.074 ms (51.8%), and τ3 = 0.015 ms (37.0%) [Fig. 2a, red solid line], suggesting that three emitting species are present in the solution of [iPr-Pybox](EuIII)(DK-NO2)3. As a negative control experiment, [iPr-Pybox](EuIII)(DK-CN)3 (reference complex) was synthesized to identify the three emitting species, where the nitro group of the β-diketone was replaced by a cyano group with a lower affinity for EuIII, but with a similar electron withdrawing capacity to the nitro group (Fig. 1). In contrast to the emission decay of [iPr-Pybox](EuIII)(DK-NO2)3 (Fig. 2a), the emission of [iPr-Pybox](EuIII)(DK-CN)3 obeys a single-exponential decay with a lifetime τ = 0.31 ms (Fig. 2b). The negative results obtained with the reference complex [iPr-Pybox](EuIII)(DK-CN)3 suggest that the three emitting species found for [iPr-Pybox](EuIII)(DK-NO2)3 are attributed to the chiral EuIII ensemble consisting of several EuIII species, where the pendant nitro group in [iPr-Pybox](EuIII)(DK-NO2)3 (monometallic EuIII species) coordinates to the EuIII center of another complex (Fig. 1). The lifetime of the longer lifetime component (τ1 = 0.26 ms (11.2%)) resembles that of the reference monometallic complex [iPr-Pybox](EuIII)(DK-CN)3 (τ = 0.31 ms), and hence the longer lifetime component (τ1 = 0.26 ms) should correspond to the monometallic complex species in the ensemble. Conversely, the shorter lifetime components (τ2 = 0.074 ms (51.8%) and τ3 = 0.015 ms (37.0%)) correspond to self-associated complexes (oligomeric EuIII species). The weak coordination between the EuIII ion and the nitro group of DK-NO2 should enhance the non-radiative processes, causing an increase in non-radiative decay rate constants for the self-associated complexes.14 Then, the EuIII ensemble was investigated by ESI mass spectrometry, where we found mass signals due to the monometallic and dimetallic EuIII species in the ESI mass of [iPr-Pybox](EuIII)(DK-NO2)3 in acetonitrile solution, HRMS [ESI-MS (positive)]: m/z calcd. for C47H38EuF9N6O14Na+ [iPr-Pybox](EuIII)(DK-NO2)3 + Na)+, 1257.14122; found 1257.14024; m/z calcd. for C94H76Eu2F18N12O28Na+ [{[iPr-Pybox](EuIII)(DK-NO2)3}2 + Na]+ 2491.30147, found 2491.29268. In addition to these signals, there are several weak mass signals assignable to EuIII complexes containing three EuIII ions, e.g., ESI-MS (positive): m/z calcd. for C97H63Eu3F24N11O34+ [[iPr-Pybox](EuIII)3(DK-NO2)8]+, 2840.08; found 2840.02. In accordance with these results, we tentatively assigned τ2 = 0.074 ms to the dimetallic EuIII species ({[iPr-Pybox](EuIII)(DK-NO2)3}2) and τ3 = 0.015 ms to the other oligomeric EuIII species (e.g., trimetallic species). We performed emission lifetime measurement at a higher concentration (1.0 × 10−4 M) of [(R)-iPr-Pybox](EuIII)(DK-NO2)3 to address this assignment (Fig. S1, ESI†), where the population of the shorter lifetime component (τ3) due to the oligomeric species increases (from 37.0% to 54.5%) with a concomitant decrease of that of the longer lifetime component due to the monomer species (from 11.2% to 6.0%). This result is consistent with the above assignment. On the other hand, a solid state sample (KBr pellet) of [iPr-Pybox](EuIII)(DK-NO2)3 exhibits an emission decay consisting of three exponential components, τ1 = 0.33 ms (33.4%), τ2= 0.18 ms (32.1%), and τ3 = 0.055 ms (34.5%) [Fig. 2c], suggesting that [iPr-Pybox](EuIII)(DK-NO2)3 also exists in the ensemble in the solid state.
exp(−t/τ3)] with τ1 = 0.26 ms (11.2%), τ2 = 0.074 ms (51.8%), and τ3 = 0.015 ms (37.0%) [Fig. 2a, red solid line], suggesting that three emitting species are present in the solution of [iPr-Pybox](EuIII)(DK-NO2)3. As a negative control experiment, [iPr-Pybox](EuIII)(DK-CN)3 (reference complex) was synthesized to identify the three emitting species, where the nitro group of the β-diketone was replaced by a cyano group with a lower affinity for EuIII, but with a similar electron withdrawing capacity to the nitro group (Fig. 1). In contrast to the emission decay of [iPr-Pybox](EuIII)(DK-NO2)3 (Fig. 2a), the emission of [iPr-Pybox](EuIII)(DK-CN)3 obeys a single-exponential decay with a lifetime τ = 0.31 ms (Fig. 2b). The negative results obtained with the reference complex [iPr-Pybox](EuIII)(DK-CN)3 suggest that the three emitting species found for [iPr-Pybox](EuIII)(DK-NO2)3 are attributed to the chiral EuIII ensemble consisting of several EuIII species, where the pendant nitro group in [iPr-Pybox](EuIII)(DK-NO2)3 (monometallic EuIII species) coordinates to the EuIII center of another complex (Fig. 1). The lifetime of the longer lifetime component (τ1 = 0.26 ms (11.2%)) resembles that of the reference monometallic complex [iPr-Pybox](EuIII)(DK-CN)3 (τ = 0.31 ms), and hence the longer lifetime component (τ1 = 0.26 ms) should correspond to the monometallic complex species in the ensemble. Conversely, the shorter lifetime components (τ2 = 0.074 ms (51.8%) and τ3 = 0.015 ms (37.0%)) correspond to self-associated complexes (oligomeric EuIII species). The weak coordination between the EuIII ion and the nitro group of DK-NO2 should enhance the non-radiative processes, causing an increase in non-radiative decay rate constants for the self-associated complexes.14 Then, the EuIII ensemble was investigated by ESI mass spectrometry, where we found mass signals due to the monometallic and dimetallic EuIII species in the ESI mass of [iPr-Pybox](EuIII)(DK-NO2)3 in acetonitrile solution, HRMS [ESI-MS (positive)]: m/z calcd. for C47H38EuF9N6O14Na+ [iPr-Pybox](EuIII)(DK-NO2)3 + Na)+, 1257.14122; found 1257.14024; m/z calcd. for C94H76Eu2F18N12O28Na+ [{[iPr-Pybox](EuIII)(DK-NO2)3}2 + Na]+ 2491.30147, found 2491.29268. In addition to these signals, there are several weak mass signals assignable to EuIII complexes containing three EuIII ions, e.g., ESI-MS (positive): m/z calcd. for C97H63Eu3F24N11O34+ [[iPr-Pybox](EuIII)3(DK-NO2)8]+, 2840.08; found 2840.02. In accordance with these results, we tentatively assigned τ2 = 0.074 ms to the dimetallic EuIII species ({[iPr-Pybox](EuIII)(DK-NO2)3}2) and τ3 = 0.015 ms to the other oligomeric EuIII species (e.g., trimetallic species). We performed emission lifetime measurement at a higher concentration (1.0 × 10−4 M) of [(R)-iPr-Pybox](EuIII)(DK-NO2)3 to address this assignment (Fig. S1, ESI†), where the population of the shorter lifetime component (τ3) due to the oligomeric species increases (from 37.0% to 54.5%) with a concomitant decrease of that of the longer lifetime component due to the monomer species (from 11.2% to 6.0%). This result is consistent with the above assignment. On the other hand, a solid state sample (KBr pellet) of [iPr-Pybox](EuIII)(DK-NO2)3 exhibits an emission decay consisting of three exponential components, τ1 = 0.33 ms (33.4%), τ2= 0.18 ms (32.1%), and τ3 = 0.055 ms (34.5%) [Fig. 2c], suggesting that [iPr-Pybox](EuIII)(DK-NO2)3 also exists in the ensemble in the solid state.
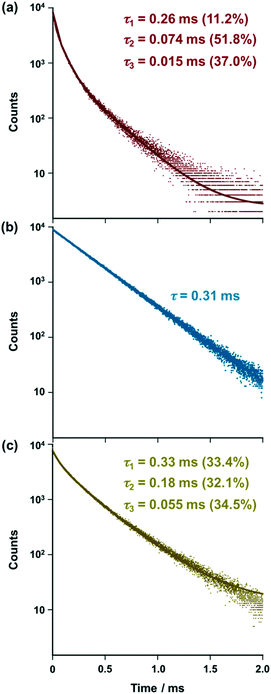 |
| | Fig. 2 Emission decay curves at 618 nm of (a) [(R)-iPr-Pybox](EuIII)(DK-NO2)3 and (b) [(R)-iPr-Pybox](EuIII)(DK-CN)3 in acetonitrile at 298 K (concentration: 1.0 × 10−5 M), and (c) that of [(R)-iPr-Pybox](EuIII)(DK-NO2)3 in the solid state (KBr pellet). Excitation-wavelength: λex = 347 nm. The solid lines show (a) and (c) multi-exponential curve fitting [I(t) = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2 exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) + A3 exp(−t/τ2) + A3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ3)] and (b) single-exponential curve fitting [I(t) = Aexp(−t/τ)]. exp(−t/τ3)] and (b) single-exponential curve fitting [I(t) = Aexp(−t/τ)]. | |
Chiroptical properties of the chiral EuIII ensemble
With these results in hand, we investigated the chiroptical properties of the chiral EuIII ensemble (vide infra). Fig. 3a shows the circular dichroism (CD) spectra of [(R)- and (S)-iPr-Pybox](EuIII)(DK-NO2)3 in acetonitrile (red and blue lines, respectively), where almost complete mirror-image CD signals are obtained with their enantiomer pairs. The chiral EuIII ensemble shows exciton-coupled biphasic (splitting) CD signals at the π–π* transition band (first absorption band) of the β-diketonate moieties (centered at λ = 355 nm),24 which is due to the induced chiral arrangement of the DK-NO2 ligands around the EuIII center by intra-complex chiral interaction with iPr-Pybox.21 Similar CD patterns to [(R)- and (S)-iPr-Pybox](EuIII)(DK-NO2)3 are observed for the reference complex [(R)- and (S)-iPr-Pybox](EuIII)(DK-CN)3 (Fig. 3b). The CD spectrum of the chiral EuIII ensemble (Fig. 3a) is the superposition of the three chiral EuIII complexes (mono-, di-, and the other oligomeric EuIII complexes). Thus, the similar CD patterns between [iPr-Pybox](EuIII)(DK-NO2)3 and [iPr-Pybox](EuIII)(DK-CN)3 (Fig. 3a and b) indicate that the additional coordination of the –NO2 group to the EuIII center has no significant effect on the exciton-coupling of the DK-NO2 ligands around the EuIII center (intra complex ligand–ligand interactions).
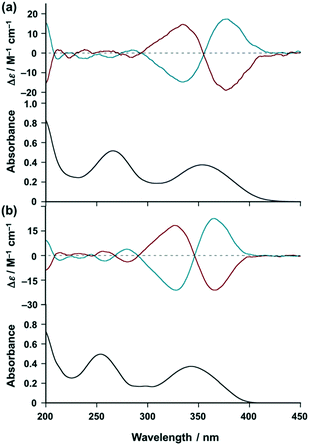 |
| | Fig. 3 Top: CD spectra of (a) [(R)-iPr-Pybox](EuIII)(DK-NO2)3 (red) and [(S)-iPr-Pybox](EuIII)(DK-NO2)3 (blue), and (b) [(R)-iPr-Pybox](EuIII)(DK-CN)3 (red) and [(S)-iPr-Pybox](EuIII)(DK-CN)3 (blue) in acetonitrile at 298 K (concentration: 1.0 × 10−5 M). Bottom: Absorption spectrum of (a) [(S)-iPr-Pybox](EuIII)(DK-NO2)3 and (b) [(S)-iPr-Pybox](EuIII)(DK-CN)3 (concentration: 1.0 × 10−5 M) in acetonitrile at 298 K. | |
On the other hand, [iPr-Pybox](EuIII)(DK-NO2)3 displays sharp emission bands due to the f–f transitions of EuIII (5D0 → 7Fn, n = 1–4) in acetonitrile, where each line splits into several lines due to the Stark effect (Fig. 4, red solid line).25 The spectral shape is different from that of the reference complex [iPr-Pybox](EuIII)(DK-CN)3 (Fig. 4, blue solid line) in terms of the Stark splitting pattern of each transition band (except for 5D0 → 7F0). Since the emission spectrum of [iPr-Pybox](EuIII)(DK-NO2)3 is the superposition of the three emitting species (mono-, di-, and the other oligomeric EuIII complexes), the observed spectral difference indicates a different crystal field environment for the EuIII between the monometallic and the oligomeric EuIII complexes. Conversely, the Stark splitting pattern of [iPr-Pybox](EuIII)(DK-NO2)3 in the solid state (Fig. 4, yellow dashed line) roughly agrees with that of the solution sample. This is consistent with the emission lifetime results for [iPr-Pybox](EuIII)(DK-NO2)3.
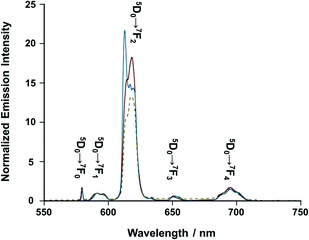 |
| | Fig. 4 Emission spectra of [(S)-iPr-Pybox](EuIII)(DK-NO2)3 (red solid line) and [(S)-iPr-Pybox](EuIII)(DK-CN)3 (blue solid line) in acetonitrile at 298 K (concentration: 1.0 × 10−5 M), and that of [(S)-iPr-Pybox](EuIII)(DK-NO2)3 in KBr pellet (yellow dashed line), where the emission intensity is normalized at the 5D0 → 7F1 transition band. Excitation-wavelength: λex = 347 nm. | |
Then, we determined the dissymmetry factors (glum) of the chiral EuIII ensemble at the 5D0 → 7F1 (magnetic dipole) transition band by using the experimental setup for the determination of the glum values.14a Since the magnetic dipole transition satisfies the magnetic-dipole selection rule, ΔJ = 0, ± 1 (except 0 ↔ 0), the magnetic dipole transition band often gives particularly large circular polarization.12Fig. 5 shows the schematic illustration of the experimental setup used in this study. In this experimental setup, a circularly polarized component (left- and right-CPL) from the sample emission converts to a linearly polarized component after passing through a rotatable λ/4 filter, which was detected by a photomultiplier tube after passing through a fixed linearly polarized plate (Fig. 5). Thus, the observed emission intensity (Iobs) oscillates every 180 degrees of the angle (θ) between the rotatable λ/4 filter and the fixed linearly polarized plate. The minimal or maximal values appear at θ = 45, 135, 225, and 315 degrees, wherein the values at θ = 45, 225 and 135, 315 degrees correspond to the right- and left-CPL intensity, respectively (IR and IL, respectively). With the use of these values, the glum values were calculated by using the equation, glum = 2(IL – IR)/(IL + IR). As a standard material for the system precision check, we have chosen the cesium tetrakis(3-heptafluoro-butylryl-(+)-camphorato) EuIII complex, Cs+[Eu((+)-hfbc)4]−. So far, Cs+[Eu((+)-hfbc)4]− shows the highest dissymmetry factor of 1.38 at the 5D0 → 7F1 transition band. This system successfully reproduced the literature value of glum = 1.38 (glum = 1.41 as determined in this study).13,14a
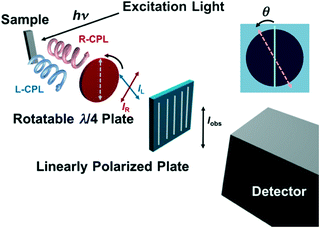 |
| | Fig. 5 Schematic illustration of the experimental setup for the determination of glum values, where “θ” denotes the angle between the rotatable λ/4 filter and the fixed linearly polarized plate. | |
Fig. 6a and b show the measurement data of [(R)- and (S)-iPr-Pybox](EuIII)(DK-NO2)3 and [(R)- and (S)-iPr-Pybox](EuIII)(DK-CN)3 at the 5D0 → 7F1 transition (λ = 594 nm) in acetonitrile solution, respectively. Almost complete mirror-image oscillation patterns are obtained with their enantiomer pairs (Fig. 6a and b, red: (R)-isomers; blue: (S)-isomers). The oscillation patterns (every 180 degrees) are well reproduced by the simulation curves provided by eqn (1) [θ in radians] with IL/IR = 0.82 for [(R)-iPr-Pybox](EuIII)(DK-NO2)3 (Fig. 6a, red line), IL/IR = 1.21 for [(S)-iPr-Pybox](EuIII)(DK-NO2)3 (Fig. 6a, blue line), IL/IR = 0.90 for [(R)-iPr-Pybox](EuIII)(DK-CN)3 (Fig. 6b, red line), and IL/IR = 1.13 for [(S)-iPr-Pybox](EuIII)(DK-CN)3 (Fig. 6b, blue line). The obtained chiroptical parameters are summarized in Table 1, in which the chiral EuIII ensemble of [iPr-Pybox](EuIII)(DK-NO2)3 exhibits a higher dissymmetry factor (|glum| = 0.19) than that of the monometallic complex of [iPr-Pybox](EuIII)(DK-CN)3 (|glum| = 0.11–0.13). The monometallic complex (n = 1) in the chiral EuIII ensemble {[(R)- or (S)-iPr-Pybox](EuIII)(DK-NO2)3}n should possess a similar luminescence dissymmetry factor (glum1) to that of the reference monometallic complex [iPr-Pybox](EuIII)(DK-CN)3, therefore the large difference in the luminescence dissymmetry factor is primarily due to the contribution of the higher degree of circular polarization in luminescence of the dimetallic and the other oligomeric EuIII species (glum2 and glum3, respectively). In such a case, the observed (overall) luminescence dissymmetry factor (goverall) can be expressed using eqn (2), where Φtotal denotes the total emission quantum yield of the chiral EuIII ensemble (Φem1 + Φem2 + Φem3). Φem1, Φem2, and Φem3 represent the emission quantum yields of the mono-, di-, and the other oligomeric EuIII species, respectively. The Φem1/Φtotal, Φem2/Φtotal, and Φem3/Φtotal terms can be calculated using eqn (3)–(5) with the emission lifetime parameters (A1–3 and τ1–3). The Φem1–3/Φtotal values were determined to be Φem1/Φtotal = 0.39, Φem2/Φtotal = 0.53, and Φem3/Φtotal = 0.077. Then, the overall luminescence dissymmetry factor (goverall) can be expressed using eqn (6), where Φem3/Φtotal could be negligible compared to Φem1/Φtotal and Φem2/Φtotal mostly due to the low emission efficiency (τ3 ≪ τ1, τ2). Consequently, |glum2| = 0.27 would be derived when assuming that the monometallic complex in the chiral EuIII ensemble has the same luminescence dissymmetry factor (glum1) as that of the reference monometallic complex (glum = 0.13). The larger luminescence dissymmetry factor (|glum2| = 0.27) of the dimetallic EuIII complex may be due to the additional coordination of the –NO2 group to the EuIII center, causing a higher degree of dissymmetry in the coordination geometry (crystal field).21
| | Iobs = IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos2(θ + π/4) + IR cos2(θ + π/4) + IR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin2(θ + π/4) sin2(θ + π/4) | (1) |
| | | goverall = (glum1Φem1 + glum2Φem2 + glum3Φem3)/Φtotal | (2) |
| | | Φem1/Φtotal = (A1τ1)/(A1τ1 + A2τ2 + A3τ3) | (3) |
| | | Φem2/Φtotal = (A2τ2)/(A1τ1 + A2τ2 + A3τ3) | (4) |
| | | Φem3/Φtotal = (A3τ3)/(A1τ1 + A2τ2 + A3τ3) | (5) |
| | | goverall = 0.39glum1 + 0.53glum2 + 0.077glum3 | (6) |
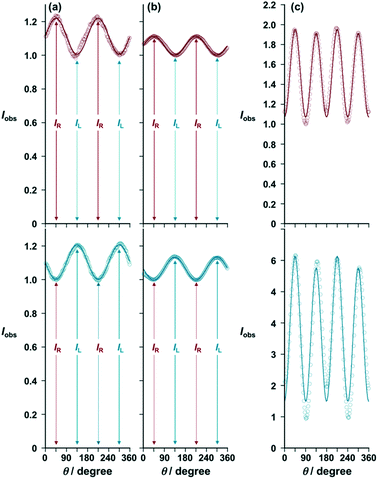 |
| | Fig. 6 Plots of emission intensity (Iobs) at λ = 594 nm vs. the angle (θ) between the rotatable λ/4 filter and the fixed linearly polarized plate for (a) [(R)-iPr-Pybox](EuIII)(DK-NO2)3 (red circles) and [(S)-iPr-Pybox](EuIII)(DK-NO2)3 (blue circles), and (b) [(R)-iPr-Pybox](EuIII)(DK-CN)3 (red circles) and [(S)-iPr-Pybox](EuIII)(DK-CN)3 (blue circles) in acetonitrile at 298 K (concentration: 1.0 × 10−5 M), where the intensity was normalized by the minimum value at θ = 45 degrees for the (S)-isomers and 135 degrees for the (R)-isomers. (c) Those of [(R)-iPr-Pybox](EuIII)(DK-NO2)3 (red circles) and [(S)-iPr-Pybox](EuIII)(DK-NO2)3 (blue circles) in the solid state (KBr pellet). Excitation-wavelength: λex = 347 nm. | |
Table 1 Chiroptical parameters (IL/IR and glum) of [(R)- and (S)-iPr-Pybox](EuIII)(DK-NO2)3 and [(R)- and (S)-iPr-Pybox](EuIII)(DK-CN)3 in acetonitrile at the 5D0 → 7F1 transition
|
|
I
L/IR |
g
lum
|
| [(R)-iPr-Pybox](EuIII)(DK-NO2)3 |
0.82 |
−0.19 |
| [(S)-iPr-Pybox](EuIII)(DK-NO2)3 |
1.21 |
0.19 |
| [(R)-iPr-Pybox](EuIII)(DK-CN)3 |
0.90 |
−0.11 |
| [(S)-iPr-Pybox](EuIII)(DK-CN)3 |
1.13 |
0.13 |
Fig. 6c shows the measurement data of [(R)- and (S)-iPr-Pybox](EuIII)(DK-NO2)3 in the solid state (KBr pellet) at the 5D0 → 7F1 transition (λ = 594 nm). The Iobs value oscillates every 90 degrees of the angle (θ) between the rotatable λ/4 filter and the fixed linearly polarized plate (minimal values at 0, 90, 180, 270, and 360 degrees; maximum values at 45, 135, 225, and 315), where no mirror-image oscillation patterns can be obtained with their enantiomer pairs [Fig. 6c, red: (R)-isomer; blue: (S)-isomer]. This shows a sharp contrast to the solution samples (Fig. 6a and b). The oscillation at every 90 degrees corresponds to the linearly polarized component from the sample emission, suggesting that the luminescence from [iPr-Pybox](EuIII)(DK-NO2)3 in the solid state (KBr pellet) contains a large linearly polarized component. The accurate dissymmetry factor could not be obtained from these measurement data due to the large contribution of the linearly polarized component. Conversely, there is no appreciable linearly polarized component in the solution samples, where the observed emission intensity (Iobs) oscillates every 180 degrees (Fig. 6a and b). The presented measuring system is typically suitable for accurate determination of the luminescence dissymmetry factor of lanthanide complexes in solutions.26
Conclusions
In conclusion, we have demonstrated how to evaluate the luminescence dissymmetry factor in an ensemble consisting of several EuIII complex species exhibiting different chiroptical properties. The chiral EuIII complex [(R)- or (S)-iPr-Pybox](EuIII)(DK-NO2)3 spontaneously forms the oligomeric EuIII species, {[iPr-Pybox](EuIII)(DK-NO2)3}n, in acetonitrile, where the pendant nitro group in [iPr-Pybox](EuIII)(DK-NO2)3 (monometallic EuIII species) coordinates to the EuIII center of another complex. Consequently, [iPr-Pybox](EuIII)(DK-NO2)3 exists in an ensemble consisting of three chiral EuIII complex species. Among them, the dimetallic chiral EuIII complex shows a larger dissymmetry factor (|glum2| = 0.27) than that of the monometallic EuIII complex, resulting in an increase of the overall luminescence dissymmetry factor of the chiral EuIII ensemble (|glum| = 0.19). The demonstrated methodology for the evaluation of circularly polarized luminescence of the chiral EuIII ensemble will open up new opportunities for developing supramolecular chiral lanthanide systems.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was partly supported by JST-PRESTO “Molecular technology and creation of new functions” (14530027), a Grant-in-Aid for Scientific Research (C) (JP26410094) from the Ministry of Education, Culture, Sports, Science and Technology, Japan and also JSPS KAKENHI Grant Number JP17H05386 (Coordination Asymmetry).
Notes and references
-
(a)
D. L. Andrews, in Photonics, Fundamental of Photonics and Physics, Wiley, Hoboken, 2015, vol. 1 Search PubMed;
(b)
Comprehensive Chiroptical Spectroscopy Instrumentation, Methodologies, and Theoretical Simulations, ed. N. Berova, P. L. Polavarapu, K. Nakanishi and R. W. Woody, Wiley, Hoboken, 2012, vol. 1 Search PubMed.
- Y. J. Zhang, T. Oka, R. Suzuki, J. T. Ye and Y. Iwasa, Science, 2014, 344, 725 CrossRef CAS PubMed.
- F. Zinna, U. Giovanella and L. Di Bari, Adv. Mater., 2015, 27, 1791 CrossRef CAS PubMed.
-
J. P. Riehl and G. Muller, in Handbook on the Physics and Chemistry of Rare Earths, ed. K. A. Gschneidner Jr., J.-C. G. Bünzli and V. K. Pecharsky, North Holland Publishing Company, Amsterdam, 2005, ch. 220, vol. 34, p. 289 Search PubMed.
-
(a) R. Carr, N. H. Evans and D. Parker, Chem. Soc. Rev., 2012, 41, 7673 RSC;
(b) C. M. G. dos Santos, A. J. Harte, S. J. Quinn and T. Gunnlaugsson, Coord. Chem. Rev., 2008, 252, 2512 CrossRef CAS;
(c) J.-C. G. Bünzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048 RSC.
-
(a) E. G. Moore, A. P. S. Samuel and K. N. Raymond, Acc. Chem. Res., 2009, 42, 542 CrossRef CAS PubMed;
(b) G.-L. Law, C. M. Andolina, J. Xu, V. Luu, P. X. Rutkowski, G. Muller, D. K. Shuh, J. K. Gibson and K. N. Raymond, J. Am. Chem. Soc., 2012, 134, 15545 CrossRef CAS PubMed.
-
(a) M. Cantuel, G. Bernardinelli, G. Muller, J. P. Riehl and C. Piguet, Inorg. Chem., 2004, 43, 1840 CrossRef CAS PubMed;
(b) G. Muller, J. P. Riehl, K. J. Schenk, G. Hopfgartner, C. Piguet and J.-C. G. Bünzli, Eur. J. Inorg. Chem., 2002, 3101 CrossRef CAS;
(c) G. Muller, B. Schmidt, J. Jiricek, G. Hopfgartner, J. P. Riehl, J.-C. G. Bünzli and C. Piguet, J. Chem. Soc., Dalton Trans., 2001, 2655 RSC;
(d) S. D. Bonsall, M. Houcheime, D. A. Straus and G. Muller, Chem. Commun., 2007, 3676 RSC.
-
(a) O. Mamula, M. Lama, S. G. Telfer, A. Nakamura, R. Kuroda, H. Stoeckli-Evans and R. Scopelitti, Angew. Chem., Int. Ed., 2005, 44, 2527 CrossRef CAS PubMed;
(b) M. Lama, O. Mamula, G. S. Kottas, F. Rizzo, L. De Cola, A. Nakamura, R. Kuroda and H. Stoeckli-Evans, Chem. – Eur. J., 2007, 13, 7358 CrossRef CAS PubMed.
-
(a) A. de Bettencourt-Dias, P. S. Barber and S. Bauer, J. Am. Chem. Soc., 2012, 134, 6987 CrossRef CAS PubMed;
(b) A. de Bettencourt-Dias, S. Viswanathan and A. Rollett, J. Am. Chem. Soc., 2007, 129, 15436 CrossRef CAS PubMed;
(c) S. Chorazy, K. Nakabayashi, M. Arczynski, R. Pełka, S.-I. Ohkoshi and B. Sieklucka, Chem. – Eur. J., 2014, 20, 7144 CrossRef CAS PubMed;
(d) K. Matsumoto, K. Suzuki, T. Tsukuda and T. Tsubomura, Inorg. Chem., 2010, 49, 4717 CrossRef CAS PubMed.
-
(a) J. I. Bruce, R. S. Dickins, L. J. Govenlock, T. Gunnlaugsson, S. Lopinski, M. P. Lowe, D. Parker, R. D. Peacock, J. J. B. Perry, S. Aime and M. Botta, J. Am. Chem. Soc., 2000, 122, 9674 CrossRef CAS;
(b) J. P. Leonard, P. Jensen, T. McCabe, J. E. O'Brien, R. D. Peacock, P. E. Kruger and T. Gunnlaugsson, J. Am. Chem. Soc., 2007, 129, 10986 CrossRef CAS PubMed.
-
(a) J. Gregoliński and J. Lisowski, Angew. Chem., Int. Ed., 2006, 45, 6122 CrossRef PubMed;
(b) J. Gregoliński, P. Starynowicz, K. T. Hua, J. L. Lunkley, G. Muller and J. Lisowski, J. Am. Chem. Soc., 2008, 130, 17761 CrossRef PubMed.
- F. S. Richardson, Inorg. Chem., 1980, 19, 2806 CrossRef CAS.
- J. L. Lunkley, D. Shirotani, K. Yamanari, S. Kaizaki and G. Muller, J. Am. Chem. Soc., 2008, 130, 13814 CrossRef CAS PubMed.
-
(a) J. Kumar, B. Marydasan, T. Nakashima, T. Kawai and J. Yuasa, Chem. Commun., 2016, 52, 9885 RSC;
(b) J. Yuasa, H. Ueno and T. Kawai, Chem. – Eur. J., 2014, 20, 8621 CrossRef CAS PubMed.
- C.-T. Yeung, W. T. K. Chan, S.-C. Yan, K.-L. Yu, K.-H. Yim, W.-T. Wong and G.-L. Law, Chem. Commun., 2015, 51, 592 RSC.
-
(a) K. S. Jeong, Y. S. Kim, Y. J. Kim, E. Lee, J. H. Yoon, W. H. Park, Y. W. Park, S.-J. Jeon, Z. H. Kim, J. Kim and N. Jeong, Angew. Chem., Int. Ed., 2006, 45, 8134 CrossRef CAS PubMed;
(b) X.-J. Kong, Y. Wu, L.-S. Long, L.-S. Zheng and Z. Zheng, J. Am. Chem. Soc., 2009, 131, 6918 CrossRef CAS PubMed.
-
(a) G. Bozoklu, C. Marchal, C. Gateau, J. Pécaut, D. Imbert and M. Mazzanti, Chem. – Eur. J., 2010, 16, 6159 CrossRef CAS PubMed;
(b) G. Bozoklu, C. Gateau, D. Imbert, J. Pécaut, K. Robeyns, Y. Filinchuk, F. Memon, G. Muller and M. Mazzanti, J. Am. Chem. Soc., 2012, 134, 8372 CrossRef CAS PubMed;
(c) X.-L. Tang, W.-H. Wang, W. Dou, J. Jiang, W.-S. Liu, W.-W. Qin, G.-L. Zhang, H.-R. Zhang, K.-B. Yu and L.-M. Zheng, Angew. Chem., Int. Ed., 2009, 48, 3499 CrossRef CAS PubMed.
-
(a) J. Gregoliński, P. Starynowicz, K. T. Hua, J. L. Lunkley, G. Muller and J. Lisowski, J. Am. Chem. Soc., 2008, 130, 17761 CrossRef PubMed;
(b) J. Lisowski, Inorg. Chem., 2011, 50, 5567 CrossRef CAS PubMed.
- In this context, we have extensively developed self-assembly in an ensemble system, see:
(a) T. Ogawa, J. Yuasa and T. Kawai, Angew. Chem., Int. Ed., 2010, 49, 5110 CrossRef CAS PubMed;
(b) J. Yuasa, A. Mitsui and T. Kawai, Chem. Commun., 2011, 47, 5807 RSC;
(c) N. Inukai, T. Kawai and J. Yuasa, Chem. Commun., 2011, 47, 9128 RSC;
(d) N. Inukai, T. Kawai and J. Yuasa, Chem. – Eur. J., 2013, 19, 5938 CrossRef CAS PubMed;
(e) N. Inukai, T. Kawai and J. Yuasa, Chem. – Eur. J., 2014, 20, 15159 CrossRef CAS PubMed;
(f) Y. Imai, T. Kawai and J. Yuasa, J. Phys. Chem. A, 2016, 120, 4131 CrossRef CAS PubMed;
(g) Y. Imai, T. Kawai and J. Yuasa, Chem. Commun., 2015, 51, 10103 RSC.
- M. Hasegawa, H. Ohtsu, D. Kodama, T. Kasai, S. Sakurai, A. Ishiia and K. Suzuki, New J. Chem., 2014, 38, 1225 RSC.
- J. Yuasa, T. Ohno, K. Miyata, H. Tsumatori, Y. Hasegawa and T. Kawai, J. Am. Chem. Soc., 2011, 133, 9892 CrossRef CAS PubMed.
- J. C. Sloop, P. D. Boyle, A. W. Fountain, W. F. Pearman and J. A. Swann, Eur. J. Org. Chem., 2011, 936 CrossRef CAS.
- Y. Hasegawa, S. Tsuruoka, T. Yoshida, H. Kawai and T. Kawai, J. Phys. Chem. A, 2008, 112, 803 CrossRef CAS PubMed.
- S. G. Telfer, N. Tajima and R. Kuroda, J. Am. Chem. Soc., 2004, 126, 1408 CrossRef CAS PubMed.
-
(a) J. Yuasa, R. Mukai, Y. Hasegawa and T. Kawai, Chem. Commun., 2014, 50, 7937 RSC;
(b) J. Yuasa, T. Ohno, H. Tsumatori, R. Shiba, H. Kamikubo, M. Kataoka, Y. Hasegawa and T. Kawai, Chem. Commun., 2013, 49, 4604 RSC;
(c) J. Yuasa, T. Nakagawa, Y. Kita, A. Kaito and T. Kawai, Chem. Commun., 2017, 53, 6748 RSC.
- The lower detection limit of the g-value by this method should be 0.01.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7me00082k |
|
| This journal is © The Royal Society of Chemistry 2018 |
 *ab
*ab
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry in methanol. Interestingly, the resulting chiral EuIII complex displays a non-single-exponential emission decay in acetonitrile solution (Fig. 2a, λem = 618 nm due to the 5D0 → 7F2 transition of EuIII).14 The decay curve can be fitted as a sum of three exponential decay components [I(t) = A1
1 stoichiometry in methanol. Interestingly, the resulting chiral EuIII complex displays a non-single-exponential emission decay in acetonitrile solution (Fig. 2a, λem = 618 nm due to the 5D0 → 7F2 transition of EuIII).14 The decay curve can be fitted as a sum of three exponential decay components [I(t) = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2
exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) + A3
exp(−t/τ2) + A3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ3)] with τ1 = 0.26 ms (11.2%), τ2 = 0.074 ms (51.8%), and τ3 = 0.015 ms (37.0%) [Fig. 2a, red solid line], suggesting that three emitting species are present in the solution of [iPr-Pybox](EuIII)(DK-NO2)3. As a negative control experiment, [iPr-Pybox](EuIII)(DK-CN)3 (reference complex) was synthesized to identify the three emitting species, where the nitro group of the β-diketone was replaced by a cyano group with a lower affinity for EuIII, but with a similar electron withdrawing capacity to the nitro group (Fig. 1). In contrast to the emission decay of [iPr-Pybox](EuIII)(DK-NO2)3 (Fig. 2a), the emission of [iPr-Pybox](EuIII)(DK-CN)3 obeys a single-exponential decay with a lifetime τ = 0.31 ms (Fig. 2b). The negative results obtained with the reference complex [iPr-Pybox](EuIII)(DK-CN)3 suggest that the three emitting species found for [iPr-Pybox](EuIII)(DK-NO2)3 are attributed to the chiral EuIII ensemble consisting of several EuIII species, where the pendant nitro group in [iPr-Pybox](EuIII)(DK-NO2)3 (monometallic EuIII species) coordinates to the EuIII center of another complex (Fig. 1). The lifetime of the longer lifetime component (τ1 = 0.26 ms (11.2%)) resembles that of the reference monometallic complex [iPr-Pybox](EuIII)(DK-CN)3 (τ = 0.31 ms), and hence the longer lifetime component (τ1 = 0.26 ms) should correspond to the monometallic complex species in the ensemble. Conversely, the shorter lifetime components (τ2 = 0.074 ms (51.8%) and τ3 = 0.015 ms (37.0%)) correspond to self-associated complexes (oligomeric EuIII species). The weak coordination between the EuIII ion and the nitro group of DK-NO2 should enhance the non-radiative processes, causing an increase in non-radiative decay rate constants for the self-associated complexes.14 Then, the EuIII ensemble was investigated by ESI mass spectrometry, where we found mass signals due to the monometallic and dimetallic EuIII species in the ESI mass of [iPr-Pybox](EuIII)(DK-NO2)3 in acetonitrile solution, HRMS [ESI-MS (positive)]: m/z calcd. for C47H38EuF9N6O14Na+ [iPr-Pybox](EuIII)(DK-NO2)3 + Na)+, 1257.14122; found 1257.14024; m/z calcd. for C94H76Eu2F18N12O28Na+ [{[iPr-Pybox](EuIII)(DK-NO2)3}2 + Na]+ 2491.30147, found 2491.29268. In addition to these signals, there are several weak mass signals assignable to EuIII complexes containing three EuIII ions, e.g., ESI-MS (positive): m/z calcd. for C97H63Eu3F24N11O34+ [[iPr-Pybox](EuIII)3(DK-NO2)8]+, 2840.08; found 2840.02. In accordance with these results, we tentatively assigned τ2 = 0.074 ms to the dimetallic EuIII species ({[iPr-Pybox](EuIII)(DK-NO2)3}2) and τ3 = 0.015 ms to the other oligomeric EuIII species (e.g., trimetallic species). We performed emission lifetime measurement at a higher concentration (1.0 × 10−4 M) of [(R)-iPr-Pybox](EuIII)(DK-NO2)3 to address this assignment (Fig. S1, ESI†), where the population of the shorter lifetime component (τ3) due to the oligomeric species increases (from 37.0% to 54.5%) with a concomitant decrease of that of the longer lifetime component due to the monomer species (from 11.2% to 6.0%). This result is consistent with the above assignment. On the other hand, a solid state sample (KBr pellet) of [iPr-Pybox](EuIII)(DK-NO2)3 exhibits an emission decay consisting of three exponential components, τ1 = 0.33 ms (33.4%), τ2= 0.18 ms (32.1%), and τ3 = 0.055 ms (34.5%) [Fig. 2c], suggesting that [iPr-Pybox](EuIII)(DK-NO2)3 also exists in the ensemble in the solid state.
exp(−t/τ3)] with τ1 = 0.26 ms (11.2%), τ2 = 0.074 ms (51.8%), and τ3 = 0.015 ms (37.0%) [Fig. 2a, red solid line], suggesting that three emitting species are present in the solution of [iPr-Pybox](EuIII)(DK-NO2)3. As a negative control experiment, [iPr-Pybox](EuIII)(DK-CN)3 (reference complex) was synthesized to identify the three emitting species, where the nitro group of the β-diketone was replaced by a cyano group with a lower affinity for EuIII, but with a similar electron withdrawing capacity to the nitro group (Fig. 1). In contrast to the emission decay of [iPr-Pybox](EuIII)(DK-NO2)3 (Fig. 2a), the emission of [iPr-Pybox](EuIII)(DK-CN)3 obeys a single-exponential decay with a lifetime τ = 0.31 ms (Fig. 2b). The negative results obtained with the reference complex [iPr-Pybox](EuIII)(DK-CN)3 suggest that the three emitting species found for [iPr-Pybox](EuIII)(DK-NO2)3 are attributed to the chiral EuIII ensemble consisting of several EuIII species, where the pendant nitro group in [iPr-Pybox](EuIII)(DK-NO2)3 (monometallic EuIII species) coordinates to the EuIII center of another complex (Fig. 1). The lifetime of the longer lifetime component (τ1 = 0.26 ms (11.2%)) resembles that of the reference monometallic complex [iPr-Pybox](EuIII)(DK-CN)3 (τ = 0.31 ms), and hence the longer lifetime component (τ1 = 0.26 ms) should correspond to the monometallic complex species in the ensemble. Conversely, the shorter lifetime components (τ2 = 0.074 ms (51.8%) and τ3 = 0.015 ms (37.0%)) correspond to self-associated complexes (oligomeric EuIII species). The weak coordination between the EuIII ion and the nitro group of DK-NO2 should enhance the non-radiative processes, causing an increase in non-radiative decay rate constants for the self-associated complexes.14 Then, the EuIII ensemble was investigated by ESI mass spectrometry, where we found mass signals due to the monometallic and dimetallic EuIII species in the ESI mass of [iPr-Pybox](EuIII)(DK-NO2)3 in acetonitrile solution, HRMS [ESI-MS (positive)]: m/z calcd. for C47H38EuF9N6O14Na+ [iPr-Pybox](EuIII)(DK-NO2)3 + Na)+, 1257.14122; found 1257.14024; m/z calcd. for C94H76Eu2F18N12O28Na+ [{[iPr-Pybox](EuIII)(DK-NO2)3}2 + Na]+ 2491.30147, found 2491.29268. In addition to these signals, there are several weak mass signals assignable to EuIII complexes containing three EuIII ions, e.g., ESI-MS (positive): m/z calcd. for C97H63Eu3F24N11O34+ [[iPr-Pybox](EuIII)3(DK-NO2)8]+, 2840.08; found 2840.02. In accordance with these results, we tentatively assigned τ2 = 0.074 ms to the dimetallic EuIII species ({[iPr-Pybox](EuIII)(DK-NO2)3}2) and τ3 = 0.015 ms to the other oligomeric EuIII species (e.g., trimetallic species). We performed emission lifetime measurement at a higher concentration (1.0 × 10−4 M) of [(R)-iPr-Pybox](EuIII)(DK-NO2)3 to address this assignment (Fig. S1, ESI†), where the population of the shorter lifetime component (τ3) due to the oligomeric species increases (from 37.0% to 54.5%) with a concomitant decrease of that of the longer lifetime component due to the monomer species (from 11.2% to 6.0%). This result is consistent with the above assignment. On the other hand, a solid state sample (KBr pellet) of [iPr-Pybox](EuIII)(DK-NO2)3 exhibits an emission decay consisting of three exponential components, τ1 = 0.33 ms (33.4%), τ2= 0.18 ms (32.1%), and τ3 = 0.055 ms (34.5%) [Fig. 2c], suggesting that [iPr-Pybox](EuIII)(DK-NO2)3 also exists in the ensemble in the solid state.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos2(θ + π/4) + IR
cos2(θ + π/4) + IR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin2(θ + π/4)
sin2(θ + π/4)




