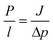Engineering microporous organic framework membranes for CO2 separations
Guangli
Yu
,
Huazhen
Rong
,
Xiaoqin
Zou
 * and
Guangshan
Zhu
* and
Guangshan
Zhu

Faculty of Chemistry, Northeast Normal University, Changchun 130024, P. R. China. E-mail: zouxq100@nenu.edu.cn
First published on 5th May 2017
Abstract
The soaring increase in CO2 emissions in the atmosphere is drastically changing our global climate and environment. To alleviate this threat, CO2 separation by membrane technology is evolving as an efficient strategy to control this emission. In the membrane process, membrane materials play a central role. Microporous materials have attracted much attention, particularly for the considerable effort in the recently developed porous organic frameworks (POFs). POFs have a promising potential in membrane applications for CO2 separation owing to the intrinsic advantages of high porosity and good processability. This review summarizes the latest advances in the development of POF membranes for CO2 separations from diverse sources. Special attention is focused on effective approaches to engineer the properties of POF membranes in terms of topological design, chemical functionalization and mixed-matrix technique, in order to enhance CO2 separation performance. The relationship between POF structures and CO2 permeability (selectivity) is also highlighted. Representative examples of POF membranes for CO2 separations from flue gas, natural gas or syngas are incorporated. A brief perspective on the future research directions in this rapidly growing field is also given.
Design, System, ApplicationThe research on microporous organic framework membranes is emerging as a brand new field of porous materials. Initial attempts have already been devoted to explore the potential in membrane application. This review focuses on the design and syntheses of microporous organic frameworks as membrane materials with multi-functionality from the pore-chemistry concept. The properties of membrane systems are engineered from both materials structures and fabrication technology. Representative examples of most recent advancements are shown to demonstrate the efficiency of these strategies to enhance CO2 separations. The present study will inspire new researches in creating novel membrane materials for efficient carbon capture. |
1. Introduction
Excessive greenhouse gas emissions pose a big threat for the sustainable development of our modern society. The negative impacts of greenhouse gases on our living circumstances, including temperature rise, sea-level increase and species extinction, have attracted widespread attention. The emission of carbon dioxide (CO2), one of the notorious greenhouse gases, is rapidly growing from energy-related and other industrial activities. Considering the increasing energy demands, it can be foreseen that CO2 emission from the major energy resource of fossil fuels will undoubtedly continue to grow. Thus, it is necessary to develop an efficient technology to rebalance the CO2 distribution and minimize the greenhouse effect. Carbon capture and storage (CCS) provides one of the feasible approaches to mitigate CO2 emissions from diverse sources. CCS technology encompasses three processes of CO2 capture and separation at stationary sources, compression and transport to an injection site, and permanent storage in geological reservoirs. The CO2 separation process is the most challenging section acquiring about 70% of the total cost of the CCS technology.1 Several technologies for CO2 separation have been proposed including cryogenic distillation, absorption, adsorption and membrane separation. The right choice of separation technology depends on the conditions of the gas stream to be treated.Membrane technology has been accepted as an effective approach for CO2 separation owing to the advantages of low cost and less energy consumption.2 The core part of membrane technology is membrane materials. Polymeric materials including rubbery and glassy polymers have occupied a big portion of the membrane materials market for CO2 separation owing to the superior physicochemical properties of excellent membrane-forming ability, processability and good mechanical strength. Unfortunately, most commonly employed polymeric membranes suffer an undesirable trade-off between permeability and selectivity (also called Robeson plot),3 which is caused by the dense state or less porous phase.4 These investigations point out that increasing material porosity is effective for improving membrane performance in order to overcome the limitation. In this regard, a lot efforts have been made to develop microporous materials for membrane application in CO2 capture or separation.2
Very recently, significant progress has been witnessed in the creation of new microporous polymers named as porous organic frameworks (POFs). POFs are built by purely organic fragments linked through covalent bonds, and the rigidness of the skeletons makes the structures porous (Fig. 1). To date, a variety of POFs with diversified structures have been synthesized, encompassing polymers of intrinsic microporosity (PIMs), covalent organic frameworks (COFs), conjugated microporous polymers (CMPs), covalent triazine-based frameworks (CTFs), hypercrosslinked polymers (HCPs), porous cages (PCs), and porous aromatic frameworks (PAFs).5–9
COFs are endowed with crystallinity as well as porosity. The crystallinity of COFs is backed by the reversible character of thermodynamically controlled reactions. PCs are molecular organic solids, demonstrating intrinsic cage-like porosity and good solubility. CTFs, as an exclusive genre of COFs, are produced by forming s-triazine rings during the cyclotrimerization of the functional nitrile groups of nitrile compounds. PIMs contain highly rigid main chains and bulky side substituents that prevent efficient chain packing, leading to porous properties. CMPs refer to macromolecules possessing microporous networks that have building blocks within the systems giving rise to π-conjugation. HCPs are amorphous polymers endued with porous structures, which are generally prepared by Friedel–Crafts alkylation chemistry. PAFs are extraordinarily high surface-area materials with permanent porosities, which are usually synthesized by forming strong C–C bonds between the aromatic monomers through coupling reactions. Most POFs bear distinguishing features of high surface area, excellent thermal stability (up to 600 °C), tuneable pore size, ordered pore structure, low framework density and multi-functionality. Because of the high porosity and small pores at the molecular level, POFs are emerging as a new candidate for membrane application.10 In recent years, extensive attempts have been made for the syntheses of new POF membranes and exploration of their applications in separations and purifications by researchers in broad fields such as chemistry, chemical engineering and materials science. The scope of this review covers the most recently developed POF materials with defined porosities. In addition, this review will summarize the latest advances in POF membranes with a focus application in CO2 separation or capture.
2. Membrane technology and POF materials
A membrane is a selective barrier, which allows one species to pass through while stopping others (Fig. 2a). Gas separation through membranes is usually driven by a pressure difference. For the evaluation of membrane performance, permeability (P) and selectivity (S) are the most important parameters (Fig. 2b). The permeability essentially measures gas molecules in moles passing across a membrane with a certain area and thickness in unit time and pressure. The selectivity is practically derived from the permeability ratio of one gas over the other. Permeability can be experimentally acquired by diffusivity (D) and adsorption ability for porous membrane and solubility for dense membrane (S) according to the following equation.| P = D × S |
In equivalence, the permeability can be also obtained theoretically. The flux can be described by Fick's law
where P/l also refers to the permeance (mol m−2 Pa−1 s−1).
For a binary gas mixture (i and j), the permeation selectivity (αij, also called separation factor) between the i and j components can be predicted from diffusion selectivity Sdiff (Di/Dj) and adsorption selectivity Sads (Δci/Δcj),
For gas separation with a porous membrane, pore chemistry plays a central role. The pore characteristics can be expressed using the terms pore size, configuration and functionality. For microporous membranes with pore sizes of 0.3–2.0 nm, the gas transport mechanisms can be catalogued into molecular sieving, Knudsen diffusion and selective surface diffusion (Fig. 2c). Pore configuration describes the pore connectivity (Fig. 2d). For gas separation, interconnected pores are prerequisite for gas diffusion. In addition, high porosity contributes largely to high gas permeability because gases can pass freely through highly connected pores in the membrane. Small pores impose strong diffusion constraints on large gas molecules when mixture gases are penetrating through the pores (Fig. 2d). Small-pore membranes can be utilized as molecular sieves to sieve a particular gas with extremely high selectivity. The surface functionality in the microporous membrane is another important parameter that governs the separation performance (Fig. 2d). The functional groups in the pores can attract gas molecules that they like or repel the gases they dislike in the mixture. For instance, the adsorption of CO2 molecules is facilitated on polar groups because of strong interactions via induced polarity or quadrupoles. The consequence of preferred adsorption is increasing the adsorption selectivity, and eventually enhancing the overall separation selectivity. In addition, the strong interaction favours close packing of CO2 molecules along the pore wall, which can improve CO2 permeability in membrane separation.
3. CO2 separations with POF membranes
The development of rational strategies to enhance CO2 capture and separation is very practical and remains a challenge. In general, the concepts for the enhancement of CO2 separations include the following: (1) structural design with high surface areas or highly interconnected pore systems, (2) tuning the pore size to efficiently screen small gases through the pores, (3) chemical functionalization of pore walls with favourable CO2 adsorption affinity, (4) mixed-matrix technique from the engineering view. In this section, we will demonstrate the feasibility of the above strategies in CO2 separations using typical examples of POF membranes.3.1 High surface areas
High-surface-area POFs are fascinating membrane materials because they contain a lot of free volume for CO2 uptake. More importantly, high surface areas usually indicate connected pore structures, which offer a highway for CO2 transport. This concept was widely adopted in the syntheses of PIM-based membranes. A typical example is PIM-TB, which was prepared using a great shape-persistent unit such as ethanoanthracene (EA) or spirobisindane (SBI).11Via the efficient formation of contorted bicyclic diamine (Tröger's base), an extreme rigidity was generated (Fig. 3a), which ensured both porosity and solubility. The high surface areas of 1028 and 745 m2 g−1 for PIM-EA-TB and PIM-SBI-TB, respectively, shed light on the highly interconnected pore systems in both PIMs (Fig. 3b). Because of the structural characteristics, PIM-TB membranes were prepared by the solution-cast method. PIM-TB membranes exhibited high gas permeability and selectivity for small gas molecules, such as CO2. For PIM-EA-TB and PIM-SBI-TB membranes with a thickness of 181 and 157 μm, CO2 permeabilities of 7140 and 2900 Barrer were measured, respectively. Moreover, CO2/N2 and CO2/CH4 selectivities of 13.6 and 10.2 for PIM-EA-TB, and 12.5 and 6.4 for PIM-SBI-TB, were obtained (Fig. 3c and d). | ||
| Fig. 3 (a) The syntheses of PIM-EA-TB and PIM-SBI-TB, (b) schematic of generated pores by contorted backbones in both PIMs, CO2 permeability and selectivity over N2 (c) and CH4 (d) for methanol-treated PIM-EA-TB for 181 μm film (1), 95 μm film (2), and the same 181 μm film after aging for 24 hours (3), and 157 μm and 128 μm films of PIM-SBI-TB (4) (adapted with permission from ref. 11). | ||
Inspired by this research, much effort has been devoted to create new POF materials with high surface areas, and the results are summarized in Fig. 4.11–33 We have found that increasing the surface areas is very effective in increasing the CO2 permeability in both cases of CO2/N2 and CO2/CH4 separations.
3.2 Ultramicroporosity
Tuning the pore size is another powerful tool for manipulating the gas-separation performances. In principle, smaller pores impose larger diffusion constraints over bigger gas molecules, which consequently in turn increase the selectivity of CO2 over other gases. The group of Pinnau engineered pore microstructures of triptycene-based polyimide membranes with pore sizes down to the ultramicroporous region.34–37 Representative examples include the syntheses of isotropic membranes of KAUST-PI-1 and KAUST-PI-2 (Fig. 5a).37 Pore size distributions showed that both materials contained a large portion of small pores with sizes below 7.0 Å (Fig. 5b and c), which originated from rigid triptycene contortion centres (Fig. 5b). As a result, KAUST-PI-1 and KAUST-PI-2 membranes served as molecular sieves for sieving CO2 molecules from the gas mixture of CO2 and CH4. In particular, a CO2/CH4 selectivity of 22.8 was measured for KAUST-PI-1 membrane, the value of which lay above the 2008 upper bound (Fig. 5d). On comparing KAUST-PI-1 and the counterparts of PTMSP and PIM-1, it was found that ultramicroporosity contributed significantly to enhance the diffusivity selectivity (Fig. 5b). From a survey of recent studies (Fig. 6), it can be concluded that micropores with dominant sizes in the regime of 3.0–7.0 Å bring great benefits in improving CO2 selectivity.17,33–56 | ||
| Fig. 5 (a) Chemical structures of KAUST-PI-1 and KAUST-PI-2, (b) scheme for gas diffusion through ultra-micropores, (c) pore-size distribution curves for PTMSP, PIM-1 and KAUST-PI-1, and (d) separation performance for CO2/CH4 gas pair for KAUST-PI-1 and KAUST-PI-2 (adapted with permission from ref. 37). | ||
3.3 Chemical functionalization
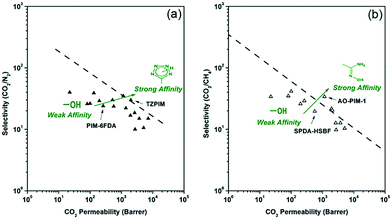
Chemical functionalization is another approach to improve CO2 separation performances using POF membranes. The functionality on the pore surface can strengthen the interaction between the framework and CO2 molecules through acid–base chemistry, electrostatic potential or induced dispersion force.The introduction of CO2-philic groups can be achieved either by judiciously chosen monomers for constructing new POFs or by post treatment of native POFs. In 2011, Guiver et al. tried to graft tetrazole groups onto a PIM-1 skeleton to increase CO2 affinity.57 During the post-treatment process, nitriles in the backbone reacted with azides to form tetrazole moieties in TZPIMs (Fig. 7a). Tetrazoles in the framework have both a basic character and acidic hydrogen, which serve as adsorption sites for CO2 molecules. The number of tetrazoles depends on the conversions (TZPIM 1–3). The processed TZPIM membranes showed super-permeable characteristics and outstanding CO2 separation performance. The data points of TZPIM-1 and TZPIM-2 in terms of CO2/N2 selectivity and CO2 permeability were beyond the upper-bound line of polymeric membranes (Fig. 7b). The enhancements in CO2/N2 selectivities for TZPIM membranes were ascribed to the preferential selective adsorption of CO2 in TZPIMs.
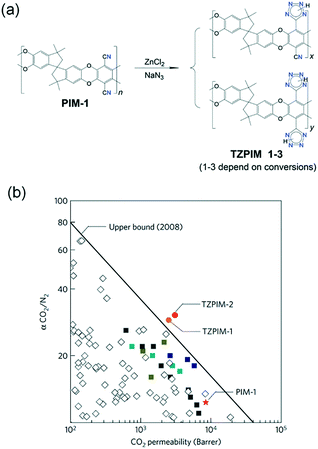 | ||
| Fig. 7 (a) Post modification process for converting PIM-1 to TZPIMs, and (b) the data of CO2 permeability and CO2/N2 selectivity of TZPIMs and other PIMs (adapted with permission from ref. 57). | ||
Recently, our group introduced a direct method for modulating surface properties of POFs by selecting monomers with functional groups. Via the condensation reaction between melamine and terephthalaldehyde, an N-rich Schiff based POF (SNW-1) was synthesised (Fig. 8a).58 In addition to the incorporation of nitrogen in the framework, narrow pores around 5.0 Å in size were simultaneously generated under the controlled co-polymerization. A huge CO2 uptake in the gas sorption measurement demonstrated the high affinity of SNW-1 toward CO2 (Fig. 8a). Both the basic nature and the microporosity of SNW-1 guaranteed its potential in CO2 separation. Several SNW-1 membranes were prepared by the spin-coating method (Fig. 8b). Gas permeation tests revealed that SNW-1 membranes possessed exceptionally high selectivity for CO2 over others, which were shown by the values of 34 and 40 for CO2/CH4 and CO2/N2 (Fig. 8c and d), respectively. The superior performance rendered SNW-1 membranes applicable in natural gas sweetening and carbon dioxide capture from flue gas.
 | ||
| Fig. 8 (a) Fragment structure of SNW-1 and the mode for CO2 interaction with the framework, (b) a representative SNW-1 membrane, and membrane separation results of (c) CO2/CH4 and (d) CO2/N2 mixtures (adapted with permission from ref. 58). | ||
The strategy of introducing CO2-philic groups in POF membranes was widely adopted by our researchers.59–65 The reported results in Fig. 9 clearly demonstrated that tailoring the pore surfaces with functionalities brought benefits in efficient CO2 capture and separation.57–72
3.4 Mixed matrix membranes
Limited POF materials are capable self-forming continuous membranes. Numerous POF materials with excellent gas adsorption capacities were prepared in the form of discrete powders. To overcome these limitations, mixed-matrix membranes (MMMs) have emerged as one of the alternative approaches that afford an enhanced gas separation performance.73In this method, the superior gas sorption of one POF material with CO2 selectivity is combined with the desirable permeability and processability of another polymer to fabricate a hybrid membrane (Fig. 10). The packed POF fillers allow building a highway for CO2 transport, responsible for its high permeability. Nevertheless, the diffusion of another gas (i.e. N2, CH4) in the matrix is relatively slow. The difference in the transport rates between these two gases results in good selectivity for CO2 over others.
 | ||
| Fig. 10 Conceptual illustration of CO2 separation using the mixed-matrix membrane with selective POF filler and polymer matrix. | ||
In this section, we demonstrate the progress in MMMs containing POF fillers with improved CO2 separation performances, the recent results of which are reported in Table 1.58,62–65,74–95 The principal fabrication procedure for producing lab-scale POF-based MMMs is similar to the one applied for the synthesis of inorganic MMMs.96 The main methods involve solution casting and fibre spinning. As for fillers, various types of POFs are explored for the construction of MMMs, such as the ones with high surfaces, superior CO2 adsorption capacities and good thermal/chemical stabilities. A functionalized PIM (cPIM-1) mixed-matrix membrane was prepared for enhancing the selectivity for CO2.74 In membrane fabrication, a carboxylated polymer of intrinsic microporosity (cPIM-1) was first prepared, followed by mixing with Matrimid to form a blended membrane. Because of the abundant carboxyl groups in the filler, a strong induced force occurs between the cPIM-1 framework and the CO2 molecule. The consequence of this interaction results in a high selectivity of CO2 over N2 (26.9) and CH4 (23.8) for a representative membrane of cPIM-1/Matrimid (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). Another example was given by a highly permeable PAF-1/PTMSP membrane.75 A series of PAF-1 and its derivatives with extremely high surface areas (829–7360 m2 g−1) were employed as the fillers. The composites of native and derived PAF-1s exhibited exceptional increase in their porosities, which resulted in ultrafast CO2 transport. For instance, high CO2 permeabilities of 28
50). Another example was given by a highly permeable PAF-1/PTMSP membrane.75 A series of PAF-1 and its derivatives with extremely high surface areas (829–7360 m2 g−1) were employed as the fillers. The composites of native and derived PAF-1s exhibited exceptional increase in their porosities, which resulted in ultrafast CO2 transport. For instance, high CO2 permeabilities of 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 and 50
400 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 Barrers were observed for PAF-1 and PAF-1-Li6C60 hybrid membranes, respectively. Moreover, the plasticization of the PAF/PTMSP membrane containing PAF-1-Li6C60 was significantly suppressed owing to the framework robustness, which was evidenced by a ∼9% reduction in the permeability after one-year of physical aging. In addition to the high-surface area PAFs, other POF materials including crystalline COFs (e.g. COF-LZU1, TpBD-COF and NUS-2) with ordered porous systems were exploited for CO2 separation membranes (Table 1). Regarding the matrix, different conventional polymers such as Ultem were adopted for the preparation of MMMs.
600 Barrers were observed for PAF-1 and PAF-1-Li6C60 hybrid membranes, respectively. Moreover, the plasticization of the PAF/PTMSP membrane containing PAF-1-Li6C60 was significantly suppressed owing to the framework robustness, which was evidenced by a ∼9% reduction in the permeability after one-year of physical aging. In addition to the high-surface area PAFs, other POF materials including crystalline COFs (e.g. COF-LZU1, TpBD-COF and NUS-2) with ordered porous systems were exploited for CO2 separation membranes (Table 1). Regarding the matrix, different conventional polymers such as Ultem were adopted for the preparation of MMMs.
| Matrix | Filler | Test conditions | CO2 permeability | CO2 selectivity | Ref. |
|---|---|---|---|---|---|
| a Reverse permeation. | |||||
| Ultem | NUS-2 | 35 °C, 2 bar | 8.7 | CO2/CH4 (13) | 62 |
| Ultem | NUS-3 | 35 °C, 2 bar | 8.1 | CO2/CH4 (11) | 62 |
| PBI-Bul | TpPa-1 COF | R. T., 20 bar | 13.1 | CO2/N2 (25) CO2/CH4 (40) | 65 |
| PBI-Bul | TpBD COF | R. T., 20 bar | 14.8 | CO2/N2 (23) CO2/CH4 (49) | 65 |
| Matrimid | cPIM-1 | 35 °C, 3.5 bar | 145 | CO2/N2 (26.9) CO2/CH4 (23.8) | 74 |
| PTMSP | PAF-1 | 25 °C, 1 bar | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
CO2/CH4 (3.0) | 75 |
| PTMSP | PAF-1-Li6C60 | 25 °C, 1 bar | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
CO2/CH4 (3.8) | 75 |
| PI | RUN | R. T., 1 bar | 1132a | N2/CO2 (6.4) | 79 |
| PPSU | PIM-1 | 35 °C, 3.5 bar | 4197 | CO2/N2 (14.9) CO2/CH4 (10.2) | 81 |
| sPPSU | PIM-1 | 35 °C, 3.5 bar | 1532 | CO2/N2 (24.7) CO2/CH4 (20.4) | 81 |
| PIM-1 | HCP | 25 °C, 2 bar | 5103 | CO2/N2 (15.1) | 82 |
| PEG | PIM-1 | 30 °C, 4 bar | 1863 | CO2/N2 (17.7) CO2/CH4 (35.4) | 83 |
| Ultem | PIM-1 | 35 °C, 3.5 bar | 51.7 | CO2/N2 (26.9) CO2/CH4 (23.2) | 85 |
| PTMSP | PAF-11 | 30 °C, 0.8 bar | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
CO2/N2 (6.3) | 86 |
| PVAm | COF-LZU1 | 25 °C, 1.5 bar | 200 | CO2/H2 (15) | 88 |
| Ultem | PIM-1 | R. T., 3 bar | 2.18 | CO2/N2 (26.2) CO2/CH4 (36.5) | 89 |
| Matrimid | PIM-1 | 35 °C, 3.5 bar | 155 | CO2/N2 (27) CO2/CH4 (28) | 90 |
| Matrimid | PIM-1 | R. T., 1 bar | 11.4 | CO2/N2 (19.6) CO2/CH4 (20.1) | 91 |
4. Conclusions and outlook
An increase in atmospheric CO2 is a worldwide concern. CO2 capture and separation using a membrane process is a promising and effective approach to reduce CO2 emissions. Herein, we have reviewed newly developed microporous membranes made of POFs. POFs are growing as a perfect candidate for membrane application in CO2 separation owing to their porous nature and good processability. To guide the researchers in the field, some fundamentals of membrane technology and POF materials have been covered, and the interplays of materials structures and gas separation performances have been discussed as well. Several strategies including topological structure design of POF membrane materials with high surface areas, ultramicroporosity, chemical functionality, as well as the mixed-matrix technique for membrane fabrication have been highlighted. Representative examples have been included to help the readers to comprehensively understand the working principles of these strategies in enhancing CO2 separation performances in terms of selectivity and permeability.Despite recent advances, several challenges still remain in the aspect of membrane science and technology for efficient CO2 separation. To further enhance CO2 capture and separation, special attention should be paid to improve the understanding of POF microstructures in order to achieve precise controls on material physicochemical properties. New characterizations and molecular simulations might provide us with useful tools for in-depth investigations of the POF structures. The limited availability of POF materials impedes the development of CO2-separation membranes. Further endeavour is still needed in the discoveries of new microporous POFs and the modifications of in-hand POFs (HCPs, CMPs, PAFs, COFs, CTFs) for designing excellent materials in CO2 separations. The phase boundary defects between POFs and polymers in the mixed matrix membranes result in poor membrane performances. New techniques are required to integrate the filler and the matrix into one entity. Currently, no general strategy can fulfil all the requirements of CO2 separation for practice, and thus it is advantageous to hybridize two or more strategies together for efficient CO2 separation in a synergistic manner. To manufacture highly stable POF membranes at a large scale will be of more industrial interest.
In summary, evidences in recent years reveals the feasibility and applicability of POF materials as CO2 separation membranes. We believe that the abovementioned strategies will accelerate the discoveries of new materials, and microporous membranes represented by POFs will expedite the formulation of membrane technology for CO2 separations.
Acknowledgements
We would like to acknowledge the financial support from the National Natural Science Foundation of China (NSFC grant no. 21531003, 21501024), the Fundamental Research Funds for the Central Universities (2412016KJ005), and Jilin Scientific and Technological Development Program (20170101198JC).Notes and references
- K. J. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T. H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS PubMed.
- P. Bernardo, E. Drioli and G. Golemme, Ind. Eng. Chem. Res., 2009, 48, 4638–4663 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
- B. D. Freeman, Macromolecules, 1999, 32, 375–380 CrossRef CAS.
- X. Feng, X. S. Ding and D. L. Jiang, Chem. Soc. Rev., 2012, 41, 6010–6022 RSC.
- S. Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548–568 RSC.
- A. I. Cooper, Adv. Mater., 2009, 21, 1291–1295 CrossRef CAS.
- A. Thomas, Angew. Chem., Int. Ed., 2010, 49, 8328–8344 CrossRef CAS PubMed.
- X. Q. Zou, H. Ren and G. S. Zhu, Chem. Commun., 2013, 49, 3925–3936 RSC.
- P. M. Budd and N. B. McKeown, Polym. Chem., 2010, 1, 63–68 RSC.
- M. Carta, R. Malpass-Evans, M. Croad, Y. Rogan, J. C. Jansen, P. Bernardo, F. Bazzarelli and N. B. McKeown, Science, 2013, 339, 303–307 CrossRef CAS PubMed.
- Y. P. Ying, D. H. Liu, J. Ma, M. M. Tong, W. X. Zhang, H. L. Huang, Q. Y. Yang and C. L. Zhong, J. Mater. Chem. A, 2016, 4, 13444–13449 CAS.
- Q. L. Song, S. Jiang, T. Hasell, M. Liu, S. J. Sun, A. K. Cheetham, E. Sivaniah and A. I. Cooper, Adv. Mater., 2016, 28, 2629–2637 CrossRef CAS PubMed.
- X. H. Ma, M. Mukaddam and I. Pinnau, Macromol. Rapid Commun., 2016, 37, 900–904 CrossRef CAS PubMed.
- B. S. Ghanem, N. B. McKeown, P. M. Budd, N. M. Al-Harbi, D. Fritsch, K. Heinrich, L. Starannikova, A. Tokarev and Y. Yampolskii, Macromolecules, 2009, 42, 7881–7888 CrossRef CAS.
- J. H. Zhou, X. Zhu, J. Hu, H. L. Liu, Y. Hu and J. W. Jiang, Phys. Chem. Chem. Phys., 2014, 16, 6075–6083 RSC.
- Z. G. Wang, X. Liu, D. Wang and J. Jin, Polym. Chem., 2014, 5, 2793–2800 RSC.
- A. D. Regno, A. Gonciaruk, L. Leay, M. Carta, M. Croad, R. Malpass-Evans, N. B. McKeown and F. R. Siperstein, Ind. Eng. Chem. Res., 2013, 52, 16939–16950 CrossRef.
- E. Chernova, D. Petukhov, O. Boytsova, A. Alentiev, P. Budd, Y. Yampolskii and A. Eliseev, Sci. Rep., 2016, 6, 31183 CrossRef PubMed.
- X. H. Ma, O. Salinas, E. Litwiller and I. Pinnau, Macromolecules, 2013, 46, 9618–9624 CrossRef CAS.
- N. Y. Du, M. M. Dal-Cin, G. P. Robertson and M. D. Guiver, Macromolecules, 2012, 45, 5134–5139 CrossRef CAS.
- I. Rose, M. Carta, R. Malpass-Evans, M. C. Ferrari, P. Bernardo, G. Clarizia, J. C. Jansen and N. B. McKeown, ACS Macro Lett., 2015, 4, 912–915 CrossRef CAS.
- M. Carta, M. Croad, R. Malpass-Evans, J. C. Jansen, P. Bernardo, G. Clarizia, K. Friess, M. Lanč and N. B. McKeown, Adv. Mater., 2014, 26, 3526–3531 CrossRef CAS PubMed.
- C. G. Bezzu, M. Carta, A. Tonkins, J. C. Jansen, P. Bernardo, F. Bazzarelli and N. B. McKeown, Adv. Mater., 2012, 24, 5930–5933 CrossRef CAS PubMed.
- Y. Rogan, L. Starannikova, V. Ryzhikh, Y. Yampolskii, P. Bernardo, F. Bazzarelli, J. C. Jansen and N. B. McKeown, Polym. Chem., 2013, 4, 3813–3820 RSC.
- E. Lasseuguette, M. Carta, S. Brandani and M. C. Ferrari, Int. J. Greenhouse Gas Control, 2016, 50, 93–99 CrossRef CAS.
- Y. L. Rogan, R. Malpass-Evans, M. Carta, M. Lee, J. C. Jansen, P. Bernardo, G. Clarizia, E. Tocci, K. Friess, M. Lanč and N. B. Mckeown, J. Mater. Chem. A, 2014, 2, 4874–4877 CAS.
- X. H. Ma and I. Pinnau, Polym. Chem., 2016, 7, 1244–1248 RSC.
- Y. B. Zhuang, J. G. Seong, Y. S. Do, W. H. Lee, M. J. Lee, M. D. Guiver and Y. M. Lee, J. Membr. Sci., 2016, 504, 55–65 CrossRef CAS.
- S. H. Li, H. J. Jo, S. H. Han, C. H. Park, S. J. Kim, P. M. Budd and Y. M. Lee, J. Membr. Sci., 2013, 434, 137–147 CrossRef CAS.
- Y. B. Zhuang, J. G. Seong, Y. S. Do, W. H. Lee, M. J. Lee, Z. L. Cui, A. E. Lozano, M. D. Guiver and Y. M. Lee, Chem. Commun., 2016, 52, 3817–3820 RSC.
- R. Swaidan, B. Ghanem, M. Al-Saeedi, E. Litwiller and I. Pinnau, Macromolecules, 2014, 47, 7453–7462 CrossRef CAS.
- X. H. Ma, B. Ghanem, O. Salines, E. Litwiller and I. Pinnau, ACS Macro Lett., 2015, 4, 231–235 CrossRef CAS.
- R. Swaidan, M. Al-Saeedi, B. Ghanem, E. Litwiller and I. Pinnau, Macromolecules, 2014, 47, 5104–5114 CrossRef CAS.
- R. Swaidan, B. Ghanem and I. Pinnau, ACS Macro Lett., 2015, 4, 947–951 CrossRef CAS.
- B. S. Ghanem, R. Swaidan, X. Ma, E. Litwiller and I. Pinnau, Adv. Mater., 2014, 26, 6696–6700 CrossRef CAS PubMed.
- B. S. Ghanem, R. Swaidan, E. Litwiller and I. Pinnau, Adv. Mater., 2014, 26, 3688–3692 CrossRef CAS PubMed.
- Q. L. Song, S. Cao, R. H. Pritchard, B. Ghalei, S. A. Al-Muhtaseb, E. M. Terentjev, A. K. Cheetham and E. Sivaniah, Nat. Commun., 2014, 5, 4813 CrossRef PubMed.
- P. M. Budd, N. B. McKeown, B. S. Ghanem, K. J. Msayib, D. Fritsch, L. Starannikova, N. Belov, O. Sanfirova, Y. Yampolskii and V. Shantarovich, J. Membr. Sci., 2008, 325, 851–860 CrossRef CAS.
- F. Y. Li, Y. C. Xiao, Y. K. Ong and T. S. Chung, Adv. Energy Mater., 2012, 2, 1456–1466 CrossRef CAS.
- Y. B. Zhuang, J. G. Seong, Y. S. Do, H. J. Jo, Z. L. Cui, J. Lee, Y. M. Lee and M. D. Guiver, Macromolecules, 2014, 47, 3254–3262 CrossRef CAS.
- M. Lee, C. G. Bezzu, M. Carta, P. Bernardo, G. Clarizia, J. C. Jansen and N. B. McKeown, Macromolecules, 2016, 49, 4147–4154 CrossRef CAS.
- M. F. Jimenez-Solomon, Q. L. Song, K. E. Jelfs, M. Munoz-Ibanez and A. G. Livingston, Nat. Mater., 2016, 15, 760–767 CrossRef CAS PubMed.
- B. S. Ghanem, N. B. McKeown, P. M. Budd and D. Fritsch, Macromolecules, 2008, 41, 1640–1646 CrossRef CAS.
- Z. A. Qiao, S. H. Chai, K. Nelson, Z. H. Bi, J. H. Chen, S. M. Mahurin, X. Zhu and S. Dai, Nat. Commun., 2014, 5, 3705 Search PubMed.
- H. Shamsipur, B. A. Dawood, P. M. Budd, P. Bernardo, G. Clarizia and J. C. Jansen, Macromolecules, 2014, 47, 5595–5606 CrossRef CAS.
- F. Y. Li, Y. C. Xiao, T. S. Chung and S. Kawi, Macromolecules, 2012, 45, 1427–1437 CrossRef CAS.
- H. B. Park, C. H. Jung, Y. M. Lee, A. J. Hill, S. J. Pas, S. T. Mudie, E. V. Wagner, B. D. Freeman and D. J. Cookson, Science, 2007, 318, 254–258 CrossRef CAS PubMed.
- N. Y. Du, G. P. Robertson, J. S. Song, I. Pinnau, S. Thomas and M. D. Guiver, Macromolecules, 2008, 41, 9656–9662 CrossRef CAS.
- Z. G. Wang, D. Wang and J. Jin, Macromolecules, 2014, 47, 7477–7483 CrossRef CAS.
- B. Ghanem, N. Alaslai, X. H. Miao and I. Pinnau, Polymer, 2016, 96, 13–19 CrossRef CAS.
- B. S. Ghanem, N. B. McKeown, P. M. Budd, J. D. Selbie and D. Fritsch, Adv. Mater., 2008, 20, 2766–2771 CrossRef CAS PubMed.
- M. Calle, C. M. Doherty, A. J. Hill and Y. M. Lee, Macromolecules, 2013, 46, 8179–8189 CrossRef CAS.
- Y. C. Xiao and T. S. Chung, Energy Environ. Sci., 2011, 4, 201–208 CAS.
- F. Alghunaimi, B. Ghanem, N. Alaslai, R. Swaidan, E. Litwiller and I. Pinnau, J. Membr. Sci., 2015, 490, 321–327 CrossRef CAS.
- S. J. Luo, J. R. Wiegand, P. Y. Gao, C. M. Doherty, A. J. Hill and R. L. Guo, J. Membr. Sci., 2016, 518, 100–109 CrossRef CAS.
- N. Y. Du, H. B. Park, G. P. Robertson, M. M. Dal-Cin, T. Visser, L. Scoles and M. D. Guiver, Nat. Mater., 2011, 10, 372–375 CrossRef CAS PubMed.
- X. Gao, X. Q. Zou, H. P. Ma, S. Meng and G. S. Zhu, Adv. Mater., 2014, 26, 3644–3648 CrossRef CAS PubMed.
- X. Zhu, C. C. Tian, S. M. Mahurin, S. H. Chai, C. M. Wang, S. Brown, G. M. Veith, H. M. Luo, H. L. Liu and S. Dai, J. Am. Chem. Soc., 2012, 134, 10478–10484 CrossRef CAS PubMed.
- N. Alaslai, B. Ghanem, F. Alghunaimi and I. Pinnau, Polymer, 2016, 91, 128–135 CrossRef CAS.
- R. Swaidan, B. S. Ghanem, E. Litwiller and I. Pinnau, J. Membr. Sci., 2014, 457, 95–102 CrossRef CAS.
- Z. X. Kang, Y. W. Peng, Y. H. Qian, D. Q. Yuan, M. A. Addicoat, T. Heine, Z. G. Hu, L. Tee, Z. G. Guo and D. Zhao, Chem. Mater., 2016, 28, 1277–1285 CrossRef CAS.
- L. B. Meng, X. Q. Zou, S. K. Guo, H. P. Ma, Y. N. Zhao and G. S. Zhu, ACS Appl. Mater. Interfaces, 2015, 7, 15561–15569 CAS.
- A. K. Sekizkardes, V. A. Kusuma, G. Dahe, E. A. Roth, L. J. Hill, A. Marti, M. Macala, S. R. Venna and D. Hopkinson, Chem. Commun., 2016, 52, 11768–11771 RSC.
- B. P. Biswal, H. D. Chaudhari, R. Banerjee and U. K. Kharul, Chem. – Eur. J., 2016, 22, 4695–4699 CrossRef CAS PubMed.
- Z. Q. Tian, S. Dai and D. E. Jiang, ACS Appl. Mater. Interfaces, 2015, 7, 13073–13079 CAS.
- H. Y. Zhao, Q. Xie, X. L. Ding, J. M. Chen, M. M. Hua, X. Y. Tan and Y. Z. Zhang, J. Membr. Sci., 2016, 514, 305–312 CrossRef CAS.
- D. Fritsch, G. Bengtson, M. Carta and N. B. McKeown, Macromol. Chem. Phys., 2011, 212, 1137–1146 CrossRef CAS.
- M. Carta, P. Bernardo, G. Clarizia, J. C. Jansen and N. B. McKeown, Macromolecules, 2014, 47, 8320–8327 CrossRef CAS.
- X. H. Ma, O. Salinas, E. Litwiller and I. Pinnau, Polym. Chem., 2014, 5, 6914–6922 RSC.
- X. H. Ma, R. Swaidan, Y. Belmabkhout, Y. H. Zhu, E. Litwiller, M. Jouiad, I. Pinnau and Y. Han, Macromolecules, 2012, 45, 3841–3849 CrossRef CAS.
- N. Y. Du, G. P. Robertson, M. M. Dal-Cin, L. Scoles and M. D. Guiver, Polymer, 2012, 53, 4367–4372 CrossRef CAS.
- N. Rangnekar, N. Mittal, B. Elyassi, J. Caro and M. Tsapatsis, Chem. Soc. Rev., 2015, 44, 7128–7154 RSC.
- W. F. Yong and T. S. Chung, Polymer, 2015, 59, 290–297 CrossRef CAS.
- C. H. Lau, K. Konstas, C. M. Doherty, S. Kanehashi, B. Ozcelik, S. E. Kentish, A. J. Hill and M. R. Hill, Chem. Mater., 2015, 27, 4756–4762 CrossRef CAS.
- J. D. Evans, D. M. Huang, M. R. Hill, C. J. Sumby, A. W. Thornton and C. J. Doonan, J. Phys. Chem. C, 2014, 118, 1523–1529 CAS.
- W. F. Yong, F. Y. Li, T. S. Chung and Y. W. Tong, J. Mater. Chem. A, 2013, 1, 13914–13925 CAS.
- Z. Z. Tian, S. F. Wang, Y. T. Wang, X. R. Ma, K. T. Cao, D. D. Peng, X. Y. Wu, H. Wu and Z. Y. Jiang, J. Membr. Sci., 2016, 514, 15–24 CrossRef CAS.
- E. Jeon, S. Y. Moon, J. S. Bae and J. W. Park, Angew. Chem., Int. Ed., 2016, 55, 1318–1323 CrossRef CAS PubMed.
- M. G. D. Angelis, R. Gaddoni and G. C. Sarti, Ind. Eng. Chem. Res., 2013, 52, 10506–10520 CrossRef.
- W. F. Yong, Z. K. Lee, T. S. Chung, M. Weber, C. Staudt and C. Maletzko, ChemSusChem, 2016, 9, 1953–1962 CrossRef CAS PubMed.
- T. Mitra, R. S. Bhavsar, D. J. Adams, P. M. Budd and A. I. Cooper, Chem. Commun., 2016, 52, 5581–5584 RSC.
- X. M. Wu, Q. G. Zhang, P. J. Lin, Y. Qu, A. M. Zhu and Q. L. Liu, J. Membr. Sci., 2015, 493, 147–155 CrossRef.
- W. F. Yong, F. Y. Li, T. S. Chung and Y. W. Tong, J. Membr. Sci., 2014, 462, 119–130 CrossRef CAS.
- L. Hao, P. Li and T. S. Chung, J. Membr. Sci., 2014, 453, 614–623 CrossRef CAS.
- A. V. Volkov, D. S. Bakhtin, L. A. Kulikov, M. V. Terenina, G. S. Golubev, G. N. Bondarenko, S. A. Legkov, G. A. Shandryuk, V. V. Volkov, V. S. Khotimskiy, A. A. Belogorlov, A. L. Maksimov and E. A. Karakhanov, J. Membr. Sci., 2016, 517, 80–90 CrossRef CAS.
- Q. L. Song, S. Cao, R. H. Pritchard, H. Qiblawey, E. M. Terentjev, A. K. Cheetham and E. Sivaniah, J. Mater. Chem. A, 2016, 4, 270–279 CAS.
- X. C. Cao, Z. H. Qiao, Z. Wang, S. Zhao, P. Y. Li, J. X. Wang and S. C. Wang, Int. J. Hydrogen Energy, 2016, 41, 9167–9174 CrossRef CAS.
- L. Hao, J. Zuo and T. S. Chung, AIChE J., 2014, 60, 3848–3858 CrossRef CAS.
- W. F. Yong, F. Y. Li, Y. C. Xiao, P. Li, K. P. Pramoda, Y. W. Tong and T. S. Chung, J. Membr. Sci., 2012, 407–408, 47–57 CrossRef CAS.
- W. F. Yong, F. Y. Li, Y. C. Xiao, T. S. Chung and Y. W. Tong, J. Membr. Sci., 2013, 443, 156–169 CrossRef CAS.
- M. X. Shan, B. Seoane, E. Rozhko, A. Dikhtiarenko, G. Clet, F. Kapteijn and J. Gascon, Chem. – Eur. J., 2016, 22, 14467–14470 CrossRef CAS PubMed.
- C. H. Lau, X. Mulet, K. Konstas, C. M. Doherty, M. A. Sani, F. Separovic, M. R. Hill and C. D. Wood, Angew. Chem., Int. Ed., 2016, 55, 1998–2001 CrossRef CAS PubMed.
- H. C. Mao and S. B. Zhang, J. Colloid Interface Sci., 2017, 490, 29–36 CrossRef CAS PubMed.
- P. Salehian, W. F. Yong and T. S. Chung, J. Membr. Sci., 2016, 518, 110–119 CrossRef CAS.
- A. Jamil, O. P. Ching and A. B. M. Shariff, Chem. Eng. Technol., 2016, 39, 1393–1405 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |