Discovery of cyclopropyl chromane-derived pyridopyrazine-1,6-dione γ-secretase modulators with robust central efficacy†
Abstract
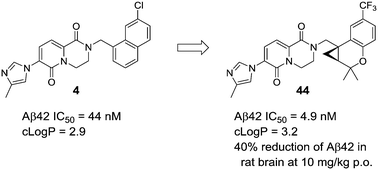
Herein we describe the discovery of a novel series of cyclopropyl chromane-derived pyridopyrazine-1,6-dione γ-secretase modulators for the treatment of Alzheimer's disease (AD). Using ligand-based design tactics such as conformational analysis and molecular modeling, a cyclopropyl chromane unit was identified as a suitable heterocyclic replacement for a naphthyl moiety that was present in the preliminary lead 4. The optimized lead molecule 44 achieved good central exposure resulting in robust and sustained reduction of brain amyloid-β42 (Aβ42) when dosed orally at 10 mg kg−1 in a rat time-course study. Application of the unpaced isolated heart Langendorff model enabled efficient differentiation of compounds with respect to cardiovascular safety, highlighting how minor structural changes can greatly impact the safety profile within a series of compounds.

 Please wait while we load your content...
Please wait while we load your content...