Applications and developments of on-chip biochemical sensors based on optofluidic photonic crystal cavities
Ya-nan
Zhang
 ab,
Yong
Zhao
ab,
Yong
Zhao
 *ab,
Tianmin
Zhou
*ab,
Tianmin
Zhou
 a and
Qilu
Wu
a and
Qilu
Wu
 a
a
aCollege of Information Science and Engineering, Northeastern University, Shenyang, 110819, China. E-mail: zhaoyong@ise.neu.edu.cn
bState Key Laboratory of Synthetical Automation for Process Industries, Shenyang, 110819, China
First published on 31st October 2017
Abstract
Photonic crystal (PC) cavities, which possess the advantages of compactness, flexible design, and suitability for integration in a lab-on-a-chip system, are able to distinguish slight variations in refractive index with only a small amount of analyte. Combined with the newly proposed optofluidic technology, PC-cavity devices stimulate an emerging class of miniaturized and label-free biochemical sensors. In this review, an overview of optofluidic PC cavities based biochemical sensors is presented. First, the basic properties of the PC, as well as the sensing principle of the PC cavity, are discussed. Second, the applications of the sensors in detecting gas, liquid, and biomolecule concentrations are reviewed, with a focus on their structures, sensing principles, sensing properties, advantages, and disadvantages. Finally, the current challenges and future development directions of optofluidic PC-cavity-based biochemical sensors are discussed.
1. Introduction
Detection of biochemical molecule concentration is necessary in many fields, including food and environment protection, health care, basic biology, and clinical diagnostics.1 At present, the most urgent task is to develop biochemical sensors that are miniature, highly sensitive, simple to use, inexpensive, and that show high precision. To realize this aim, some novel methods that take full advantage of advanced microfabrication technology, optical theory, and materials science to integrate biochemical sensors in a lab-on-a-chip system have flourished over recent years.2,3 Such methods include surface plasmon (SPR) resonator,4 optical resonator,5–7 optical grating,8 laterally-illuminated photonic crystal (PC) cavity,9–12 and normally-illuminated PC slab.13 Some commercial products based on the above technologies are listed in Table 1. These products inherit the favorable characteristics of optical sensors, including safety in flammable and explosive environments, immunity to electromagnetic interference, remote monitoring, and rapid response. In particular, due to the prominent advantages of PCs, such as their ultra-compact size (of the order of tens to hundreds of microns squared), small amount of analyte required, high spectral sensitivity, and integration capability, considerable attention has been devoted to the PC in relation to biochemical sensing applications.14 In comparison, the normally-illuminated PC slab sensor, with a PC resonant reflector surface as the sensor head, can be illuminated/detected at a normal incidence.15 By introducing an optical fiber-coupled traveling-wave semiconductor amplifier as the gain media, it enables a high degree of multiplexing, a high-quality factor, and high refractive-index sensitivity.11 A detailed introduction of biochemical sensors based on a normally-illuminated PC slab can be found in ref. 16. In contrast, in laterally-illuminated PC-cavity sensors, the sensing heads are connected with two optical fibers at the lateral sides of the PC, and they feature a high degree of compactness. Furthermore, different PC-cavity sensors can share one simple optical source and set of detection instruments, thus offering the possibility of integrating several sensors on one chip. In this review, the use of laterally-illuminated PC cavities as the biochemical sensing platforms will be discussed, mainly concerning their applications in lab-on-a-chip systems.| Technology | Manufacturer | Instruments | Integration | Response time | Website |
|---|---|---|---|---|---|
| SPR | Biosensing Instruments | BI-4500 | 5 channels | 4 ms | http://www.biosensingusa.com |
| GE Healthcare/Biacore | Biacore 8K | 8 channels (384 well microplates) | 2–15 min | http://www.proteins.gelifesciences.com | |
| NanoSPR | NanoSPR9 | 2 channels | 0.2 s | http://www.nanospr.com | |
| Reichert Analytical | Reichert4SPR | 4 channels | — | http://www.reichertspr.com | |
| BioNavis | 420A ILVES | 4 (96 well microplates) | — | http://www.bionavis.com | |
| PC | Cunningham Group and SRU Biosystems | — | 1536 well microplates | — | http://www.nano.ece.illinois.edu |
| Optical grating | Axela | DotLab | — | — | http://www.axelabiosensors.com |
| Resonant gratings | Corning | EPIC system | 384 well microplates | 15 s | http://www.corning.com |
| Ring resonator | Genalyte | Maverick | 128 rings | 15 min | http://www.genalyte.com |
In contrast with the conventional optical cavity that has a closed structure with its cavity layer sandwiched between two high-reflected surfaces,17 the PC configuration forms a unique open cavity, which allows its cavity layer (sensing layer) to be easily functionalized and directly exposed to microfluidics for sensing.18 It has been demonstrated that the strong confinement of light in the PC defect cavity might incur some very narrow resonant modes, both in the transmission and reflection spectrum of the PC cavity, which yields heavy perturbation when chemical- or biomolecules are captured on the cavity surface. Therefore, both a low detection limit and a high measurement sensitivity can be obtained for such a PC-cavity-based biochemical sensor.19
On the other hand, optofluidic has become a new photonic branch in biochemical sensing,20 which integrates nanophotonic on the manipulation of photons at the scale of an optical wavelength, with microfluidic on the control of fluids at the micron scale.21 In addition, the large fraction of air holes in the PC cavity is a natural candidate for storing fluids. Therefore, the combination of the PC cavity and the optofluidic can further promote the sensing applications of the on-chip integrated PC cavity,22,23 and make it possible for the optofluidic PC-cavity-based sensor to be sensitive to external chemical- or bio-stimuli, such as gas, bulk liquid concentration, protein, DNA, avidin, nanoparticles, and cancer cells.
In this work, an overview of the on-chip biochemical sensors based on the optofluidic PC cavity is given in detail. The rest of this paper is organized as follows: in section 2, the theoretical background behind the PC cavity is analyzed. In section 3, a discussion of the way in which PC cavities are used as biochemical sensors, along with their structures, sensing principles, and sensing properties, is presented. In section 4, the existing challenges and future research directions of the optofluidic PC cavity for biochemical sensing applications are put forward. Finally, in section 5, we draw a brief conclusion and discuss future prospects.
2. Basic properties and sensing principle of the PC cavity
A PC is usually formed by periodically arranging air holes in high-refractive-index dielectric materials (e.g. glass or silicon). The periodicity of the PC can be classified into one, two, and three spatial dimensions. The pattern in which the dielectric materials are distributed is called a lattice. The constructive and destructive interferences that are generated from the various reflections and refractions of photons within these contrasting dielectric materials, and more importantly at the boundaries between them, give rise to some photonic pass and stop bands, similar to the electronic band gaps in semiconductors. The propagation of light within the wavelength range of the photonic band gap (PBG) is forbidden.24 A small perturbation of the refractive index distribution (or arrangement) of dielectric materials in the PC can provide an impressive amount of control over the propagation of light, and thus enable the development of PC sensing technologies.25 By applying electromagnetic perturbation theory, the influence of the refractive-index variation can be given as:26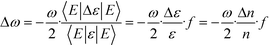 | (1) |
From this equation, it is clear that the frequency change of an optical mode in the PC depends linearly on both the refractive-index variation and the filling fraction. Since the change in refractive index can typically reflect the properties of the analyte being detected, researchers have sought to enhance the sensing sensitivity of the PC by localizing the electric field energy only to the region where analyte interrogation will occur.27 It is well known that once defects are introduced into a PC, the periodicity of its dielectric function will be broken, making it possible to guide and manipulate the way that the light interacts with the artificial PC at the scale of an optical wavelength. In particular, when certain point defects are introduced in the orderly arranged lattices of the PC, a PC cavity that is potentially surrounded by reflecting walls may be formed. As long as this new space supports an optical mode whose frequency is located inside the PBG, light can be “trapped” there for an extended number of field oscillation cycles, thus bringing strong spatial and temporal light confinement and long photon lifetime (namely, high-quality factor Q) in the PC cavity.28
As shown in Fig. 1, the typical point defected structures of the PC cavity can be classified into four types: 1) Hm-typed: modifying one or more lattice points to create a small space that is potentially surrounded by reflecting walls;29,30 2) Ln-typed: removing some lattice points in one line to form a cavity;31–33 3) ring-typed: removing some lattice points to form one or more ring-typed defects and generate some localized optical modes that resonated in these rings;34,35 4) hetero-typed: spanning a large number of lattice points with different lattice constants, holes sizes, holes locations, or shapes to generate resonant modes.36,37 In many cases, the PC waveguide, which is formed by removing a row of air holes, enables a transmission spectrum to be recorded after light is transmitted through the PC cavity. In general, the straight waveguide is used for routing light to an Ln-typed, ring-typed, or hetero-typed cavity, and side-coupling light to an Hm-typed or Ln-typed cavity.
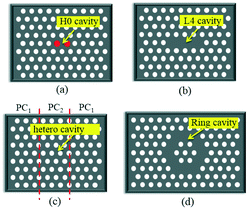 | ||
| Fig. 1 Schematic structures of different PC cavities. (a) H0 cavity, (b) L4 cavity, (c) hetero cavity and (d) ring cavity. | ||
As for sensing applications, the enhanced interaction effect gives rise to an optical mode of the PC cavity whose resonant wavelength is highly sensitive to the local refractive-index variation in its surrounding medium. This, in turn, makes it useful for highly sensitive sensing purposes, since the analyte of interest within the PC cavity can greatly change the local refractive index. In this regard, PC cavities are widely utilized to develop sensing platforms for multiple applications in chemical sensing and biosensing. Briefly, a biochemical interaction (e.g., binding) on the PC cavity may cause a change in the effective refractive index of air holes in the PC cavity, which will then result in a shift of the resonant wavelength that is proportional to the concentration of the biochemical target. To obtain the wavelength shift, almost all the published biosensor configurations based on the PC cavity share the same detection instrument configurations, in which a low-cost broadband source and a coupling lens are used to couple light into the PC waveguide, with an optical spectrum analyzer or a power meter on the other side of the PC waveguide to obtain the resonant spectrum. In particular, different PC cavities can be integrated on one chip, and each PC cavity will independently shift its resonant wavelength in response to corresponding changes in measurement signals, without perturbing the others. As a result, all the wavelength shifts of the different PC cavities that carried the information of different measurement signals can be interrogated simultaneously by using one optical spectrum analyzer, which provides an ideal platform for realizing ultra-compact lab-on-a-chip applications with dense arrays of functionalized spots for multiplexed sensing.18,38
3. Sensing applications
Recent advances in optofluidics technology hold promise for providing compact and portable platforms in biochemical sensing applications to realize rapid, highly sensitive, label-free, and on-chip detection of biochemical molecules. The PC cavity has been used to detect changes in the refractive index of liquids or gases and also changes in the refractive index of the sensor surface induced by adsorption of either chemical or biological molecules. Combined with the considerable benefits of the PC-cavity-based sensor, optofluidic PC-cavity sensors have been broadly employed to detect multiple biochemical targets, such as protein, DNA, avidin, nanoparticles, and cancer cells. Here, we summarize the typical applications of the optofluidic PC cavity in on-chip biochemical sensors. For each sensing application, the structural configuration, sensing principle, sensing properties, advantages and disadvantages will be reviewed in detail.3.1 On-chip detection of gas concentration
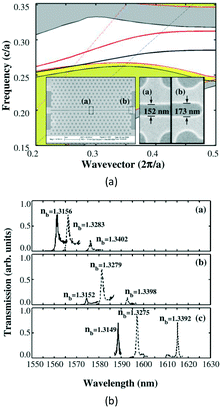
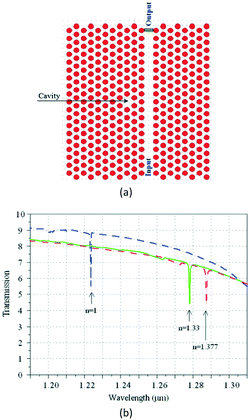
With the increasing depletion of coal, oil, and other fossil fuels, measurement of gas concentration has assumed increasing importance.39 In particular, some trace gases need to be measured in a narrow space, which immediately invalidates most gas sensors. The periodic air hole microstructure of a PC cavity is a natural candidate for housing gas analytes, so it is easy to think that the refractive index of the air hole, as well as the resonant wavelength of the PC cavity, will change with the concentration variation of an infiltrated gas. In comparison with traditional gas sensors, PC-cavity-based gas sensors exhibit the merits of both PC-cavity sensors and optical gas sensors, and the volume can be drastically reduced. By using this measurement principle, Sünner et al. proposed a hetero-typed PC cavity by modulating the radii of the first row of air holes adjacent to the waveguide.40 While this sensor structure could be used to identify vacuum, nitrogen, and SF6, its refractive-index sensitivity was only 80 nm per refractive-index units (RIU). Based on this, Jágerská et al. improved the sensitivity by introducing an air slot in a hetero-typed PC cavity.41 An experimental sensitivity up to 510 nm RIU−1 and a detection limit higher than 1 × 10−5 RIU were demonstrated. However, to obtain high-quality factor and high sensitivity, the structure of the hetero-typed PC cavity needs to be carefully optimized and finely tuned, resulting in a low tolerance to fabrication deviation. To resolve this problem, Li et al. recently proposed an Ln-slot PC cavity for gas-sensing applications,42 as shown in Fig. 2. Beyond the simple structure and high fabrication tolerance of this PC cavity, a quality factor exceeding 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and sensitivity up to 421 nm RIU−1 were experimentally demonstrated, potentially greatly extending its application in gas sensors. However, it should also be mentioned that the refractive index of gas is relatively low (∼1.0 RIU) and the corresponding refractive-index variation due to the gas concentration change is usually lower than 10−4 RIU. Furthermore, the variations of any other gases or environmental parameters can all result in the refractive-index variation of the air hole in the PC cavity. Therefore, the above proposed gas sensors based on PC cavity remain conceptual, making them unsuitable for identifying the concentration of target gas, and creating very poor sensitivities compared with other conventional gas sensors.
000 and sensitivity up to 421 nm RIU−1 were experimentally demonstrated, potentially greatly extending its application in gas sensors. However, it should also be mentioned that the refractive index of gas is relatively low (∼1.0 RIU) and the corresponding refractive-index variation due to the gas concentration change is usually lower than 10−4 RIU. Furthermore, the variations of any other gases or environmental parameters can all result in the refractive-index variation of the air hole in the PC cavity. Therefore, the above proposed gas sensors based on PC cavity remain conceptual, making them unsuitable for identifying the concentration of target gas, and creating very poor sensitivities compared with other conventional gas sensors.
 | ||
| Fig. 2 Microscopic structure (a) and gas sensing properties (b) of Ln slot PC cavity.42 | ||
In order to address these problems and capitalize on the advantages of the ultra-compact and high refractive-index sensitivity of the PC-cavity sensor, our group first proposed a gas concentration sensor using a cryptophane-E-infiltrated PC cavity,43 as shown in Fig. 3. The refractive index of the cryptophane-E would only change with variation in the concentration of methane, which would then induce a resonant wavelength shift of the PC cavity. By combining the selective adsorption property of the cryptophane-E to methane and the excellent resonant properties of the PC cavity, the resonant spectrum of the PC cavity would sharply shift with the change in concentration of methane, allowing high precision and highly sensitive measurement of methane concentration. Finally, a theoretical detection limit of 697.35 ppm for sensing of methane concentration was achieved. This technology provides a new direction for the gas sensor based on PC cavity. Next, we further proposed a gas-sensing system based on the fiber loop ring-down technique to realize the high-precision demodulation of the output resonant spectrum of the PC cavity. In particular, slow light was introduced to enhance the refractive-index sensitivity of the PC cavity.44 For this design, the detection limit was improved to 2.37 ppm. However, it should be mentioned that the sensing properties of this sensor are also not as good as some conventional gas sensors, whose detection limit may be as low as 1 ppb.45 At the same time, Liapis et al. proposed a compact and high-resolution on-chip spectrometer by using a thermally tuned PC cavity, as shown in Fig. 4. This chip-scale spectrometer could be used to measure the absorption spectra of both acetylene and hydrogen cyanide in the 1550 nm spectral band.46 However, the detection limit of gas concentration was not given. From the above schemes, it is found that the PC cavity can be used for on-chip detection of gas concentration, which behaves in miniaturized size. However, the sensitivity and precision of concentration measurement still need to be improved.
 | ||
| Fig. 3 Schematic structure (a) and gas sensing properties (b) of a cryptophane E infiltrated PC cavity. Adapted from ref. 43, copyright 2015. | ||
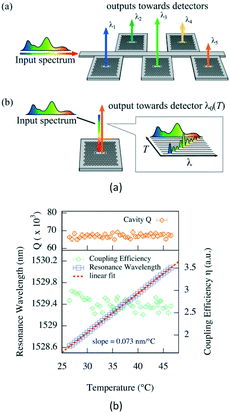 | ||
| Fig. 4 Schematic structure (a) and resonant properties (b) of thermally tuned PC cavity. Adapted from ref. 46, copyright 2016. | ||
3.2 On-chip detection of liquid concentration
The detection of liquid concentration also utilizes the refractive-index variation of the air hole, by infiltrating the test liquid into certain holes of the PC cavity. As a result, the resonant wavelength of the PC cavity will change with variations in the liquid concentration. To increase the measurement sensitivity, many types of PC cavity structures have been proposed.The first proposal is the hetero-typed PC cavity. In 2009, Di Falco et al. fabricated a hetero-typed PC cavity by gradually adjusting the width of the air slot,47 as shown in Fig. 5. This peculiar cavity possesses a high-quality factor of about 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and enables the detection of a slight refractive-index change with high sensitivity up to 1538 nm RIU−1. However, the gradual adjustment of slot width is very difficult, which will increase the complexity and cost of PC-cavity fabrication.
000, and enables the detection of a slight refractive-index change with high sensitivity up to 1538 nm RIU−1. However, the gradual adjustment of slot width is very difficult, which will increase the complexity and cost of PC-cavity fabrication.
 | ||
| Fig. 5 Schematic structure (a) and liquid concentration-sensing properties (b) of one hetero-typed PC cavity. Adapted from ref. 47, copyright 2009. | ||
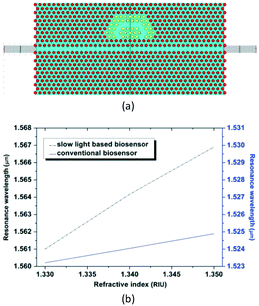
The second proposal is the ring-typed PC cavity. In 2012, Hosseinibalam et al. proposed another PC cavity, which was composed of a half-ring cavity that was side-coupled to an optofluidic slow light PC waveguide,48 as shown in Fig. 6. Simulation results demonstrated that the sensitivity to refractive-index change could be increased from 77 nm RIU−1 to 293 nm RIU−1 when slow light was introduced.
 | ||
| Fig. 6 Schematic structure (a) and liquid concentration sensing properties (b) of one optofluidic based ring-typed PC cavity. Adapted from ref. 48, copyright 2012. | ||
Coincidentally, Lai et al. have demonstrated experimentally that in a side-coupled cavity-waveguide configuration, group velocity of the propagating mode in the coupled waveguide plays an important role in enhancing the sensitivity.49 Taking a linear L13 PC cavity for example, the sensing sensitivity would increase from 57 nm RIU−1 to 66 nm RIU−1 when the group index in the coupled waveguide was increased from 10.2 to 13.2, with nearly the same quality factor of 7000. However, engineering for the highest sensitivity in such a planar-integrated sensor requires careful design of the PC structure to enhance the group index of slow light, which still needs to be researched in the future.
On the basis of the half-ring cavity, Ho et al. investigated another PC cavity which comprised three hexagonal nano-rings.50 It was demonstrated that the symmetrical resonance output of the three PC cavities could be used for simultaneous sensing of three variables at same input frequency.
The third proposal is the Ln-typed PC cavity. In 2013, Olyaee et al. proposed a PC cavity by introducing waveguides and a cavity into the periodic PC structure.51 The waveguides were obtained by eliminating two groups of air holes, and the cavity was formed by changing the dimensions of the air holes. The simulation results showed that the resonant wavelength of the PC cavity would shift to longer wavelengths with trapping of biochemical molecules, with a sensitivity of 63.1 nm RIU−1. This kind of PC cavity has relatively high transmissivity, but its refractive-index sensitivity is low.
Then, in 2014, Najafgholinezhad et al. proposed another shoulder-coupled PC cavity, which was created by eliminating a row of holes and substituting a hole with a different radius.52 As a result, the designed structure had a high-quality factor of about 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and a sensitivity of approximately 141.67 nm RIU−1. Furthermore, the presence of fluid with a negative thermo-optic coefficient could balance the thermal drift of the main material of the PC cavity, such as silicon, resulting in a low-temperature sensitivity of about −0.0142 nm °C−1.
000 and a sensitivity of approximately 141.67 nm RIU−1. Furthermore, the presence of fluid with a negative thermo-optic coefficient could balance the thermal drift of the main material of the PC cavity, such as silicon, resulting in a low-temperature sensitivity of about −0.0142 nm °C−1.
In the same year, Zhou et al. also improved this structure by modifying the number and sizes of the air holes nearest to the PC cavity,53 as shown in Fig. 7. Simulation results demonstrated that the refractive-index sensitivity could be up to 131.70 nm RIU−1. In addition, through changing the number of functionalized holes, the sensitivity could also be increased.
 | ||
| Fig. 7 Schematic structure (a) and liquid concentration sensing properties (b) of one Ln-typed PC cavity.53 | ||
On this basis, Huang et al. proposed a ring-slot PC cavity, whose resonant properties could be controlled by adjusting the width of the ring-slot.54 Simulation results demonstrated that a refractive-index sensitivity of 160 nm RIU−1 and detection limit of 8.75 × 10−5 RIU could be obtained, when the width of the ring-slot was 0.28a. The width and refractive index of the ring-slot have a great influence on the resonant properties of the PC cavity. Besides, the ring-slot is eminently suitable for housing the liquid to be measured. Therefore, this ring-slot PC cavity can be applied well in sensing the concentration of the liquid.
The fourth proposal is the Hm-typed PC cavity. In 2009, Yang et al. presented nanoscale PC sensor arrays on a monolithic substrate, which could be used as an optofluidic architecture for performing highly parallel detection of liquid concentration.55 The architecture consisted of arrays of lattice-shifted resonant cavities side-coupled to a single PC waveguide, as shown in Fig. 8. Each resonant cavity was formed by shifting the positions of two air holes. Simulation results demonstrated that a refractive-index sensitivity of 115.60 nm RIU−1 could be achieved and the refractive-index detection limit was approximately 8.65 × 10−5 RIU for this device. Besides, by setting different cavity spaces, the resonant peaks of the different PC cavities were different, and each independent resonant peak would shift with the refractive-index change of the corresponding cavity. The fabrication is relatively simple, but the detection sensitivity still needs to be increased.
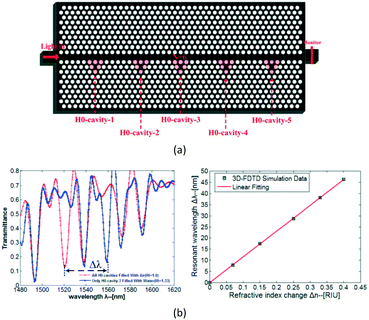 | ||
| Fig. 8 Schematic structure (a) and liquid concentration sensing properties (b) of one Hm-typed PC cavity. Adapted from ref. 55, copyright 2011. | ||
Then, in 2014, Liu et al. proposed radius-graded PC sensor arrays applied on a nanoscale optical platform for refractive-index sensing.56 Two L3 cavities and two H1 cavities were multiplexed and interlaced on both sides of a PC W1 waveguide. In response to the refractive-index changes of air holes surrounding the PC cavities, four interlaced and symmetrical cavities were shown to independently shift their resonant wavelength without crosstalk. The simulation results demonstrated that the refractive-index sensitivity of the sensor array could vary from 66.67 to 136.67 nm RIU−1 when the number of functionalized air holes was changed from 4 to 21. This design allows different cavities multiplexed on both sides of the waveguide. Meanwhile, the radius-graded PC with more symmetrical and interlaced cavities is better for large integration in the sensor arrays.
By utilizing this structure, we have proposed a new method for the simultaneous measurement of liquid concentration and temperature.57 In this design, two cascaded cavities (H0 cavity and H1 cavity) were separately located adjacent to one waveguide. A standard liquid with a fixed and known concentration was infiltrated in the defected holes of the H1 cavity, and the measured liquid with unknown concentration was infiltrated in the defected holes of the H0 cavity. The two cavities had two different and independent resonant dips, which could be simultaneously observed at the output spectrum of the waveguide. Furthermore, the variation of the test liquid concentration and the variation of temperature could all cause the wavelength shifts of the two resonant dips. However, the shift sensitivities are different. Therefore, according to the dual-wavelength matrix method, the liquid concentration measurement with a resolution of 9.4322 ppm and the temperature measurement with a resolution of 0.0136 °C were simultaneously obtained by measuring the shifts of the two resonant dips.
3.3 On-chip detection of biomolecule concentration
In 2007, Lee et al. reported that an H0-typed PC cavity with only one spot defect (see Fig. 9) was able to detect a protein molecule as small as 2.5 fg, while the active sensing volume could be as low as 0.15 μm2.58
 | ||
| Fig. 9 Schematic structure of H0-typed PC cavity for protein concentration measurement. Adapted from ref. 58, copyright 2007. | ||
Then, in 2009, Dorfner et al. proposed an L3-typed PC cavity (see Fig. 10) for the sensing of bovine serum albumin.59 The cavity was coupled to a ridge waveguide that allowed the introduction of a fluid flow cell on a chip. A response of ∂λ/∂c = (4.54 ± 0.66) × 105 nm M−1 was measured, which lead to a detection limit as good as ∂m = 4.0 ± 0.6 fg or ∂m/∂A = (4.9 ± 0.7) × 102 pg mm−2 in the sensitive area.
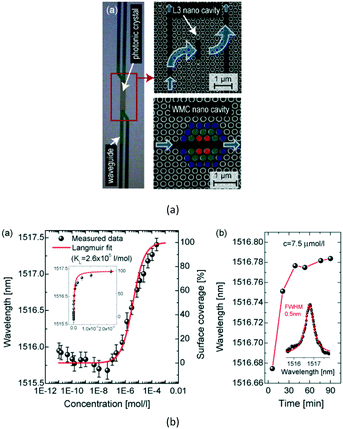 | ||
| Fig. 10 Schematic structure (a) and liquid concentration sensing properties (b) of one Hm-typed PC cavity. Adapted from ref. 59, copyright 2009. | ||
Later in 2011, Pal et al. proposed a point-defect PC cavity for label-free and error-corrected detection of human immunoglobulin (IgG) molecules.60 Experimental results showed that the structure had a refractive-index detection limit of 10−2 RIU and a biosensing sensitivity of 2.3 ± 0.24 × 105 nm M−1, with an achievable lowest detection limit of 1.5 fg for the human IgG molecule. Additionally, the experimental results demonstrated that the PC cavity was specific in IgG detection and provided a concentration-dependent response consistent with Langmuir behavior. This PC-cavity device shows outstanding potential as a microscale label-free error-correcting sensor, and may have future utility in ultrasensitive multiplex devices.
In 2012, Lai et al. experimentally demonstrated that an Ln-type PC-cavity-based resonant sensor coupled to a PC waveguide could be used for protein sensing.61 It was demonstrated that increasing the length of the PC cavity could enhance the quality factor of the resonance by an order of magnitude and increase the resonant wavelength shift, while retaining compact device characteristics. As a result, a high-quality factor of Q ∼26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 and a detection limit down to 15 ng ml−1 and ∼110 pg mm−2 in protein sensing were experimentally demonstrated.
760 and a detection limit down to 15 ng ml−1 and ∼110 pg mm−2 in protein sensing were experimentally demonstrated.
Then, in 2015, Yang et al. fabricated a high sensitive L13-type PC cavity with nanoholes, and measured its sensitivity to pancreatic cancer biomarkers in patient serum samples.62 As a result, a sample with 0.03 pM concentration was experimentally detected, which was 10-times lower than the dilution achieved with enzyme-linked immunosorbent assay (ELISA).
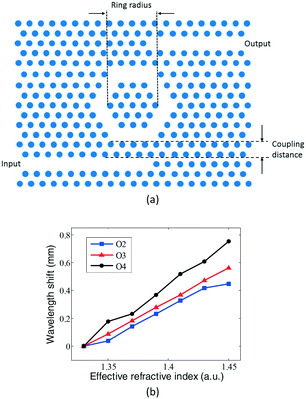 | ||
| Fig. 11 Schematic structure (a) and sensing property (b) of ring-typed PC cavity for DNA weight measurement. Adapted from ref. 63, copyright 2010. | ||
In 2013, Olyaee et al. proposed an H1-typed PC cavity (see Fig. 12) for the detection of biomaterials such as DNA molecules and proteins.65 A quality factor and sensitivity of about 4000 and 1.63 nm fg−1, respectively, were obtained. Furthermore, the bulk refractive-index sensitivity for this structure was about 165.45 nm RIU−1. Besides, to enhance the number of detected target molecules, a multichannel biosensor was designed by lattice shifting a single hole around the cavity. Each channel had a different resonant cavity wavelength and the filling of analyte in selected holes independently caused a resonant wavelength shift.
 | ||
| Fig. 12 Schematic structure (a) and sensing property (b) of H1-typed PC cavity for DNA weight measurement. Adapted from ref. 65, copyright 2013. | ||
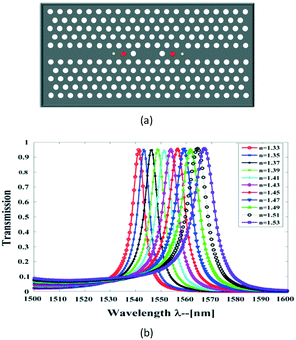
Then, in 2015, these researchers further presented a diamond-shaped sensor based on a PC nano-ring cavity.66 The ring cavity with two end waveguides on both sides, were placed in the middle of the PC structure, as shown in Fig. 13. Simulation results demonstrated that the resonant wavelength shift was linearly proportional to the refractive-index variation in the refractive-index range of 1.33–1.54. The quality factor and the sensitivity of the biosensor were obtained at about 3700 and 3.4 nm fg−1, respectively. The minimum detectable biomolecule weight in a sensing hole for a diamond-shaped nano-ring resonator was derived as 0.029 fg.
 | ||
| Fig. 13 Schematic structure (a) and sensing property (b) of ring-typed PC cavity for protein concentration measurement. Adapted from ref. 66, copyright 2015. | ||
By combining the spatial confinement of the optical field provided by a slot waveguide with the temporal confinement of the optical field in a PC cavity, Scullion et al.68 first demonstrated the possibility of the slot PC cavity (see Fig. 14, lattice constant 490 nm, cavity period 460 nm, hole radius 135 nm, and slot width 120 nm) in the detection of dissolved avidin concentration as low as 15 nM or 1 μg ml−1, with a sensing area of only 2.2 μm2. The high sensitivity over an extremely small area is due to the strong modal overlap with the analyte enabled by the slotted waveguide cavity geometry.
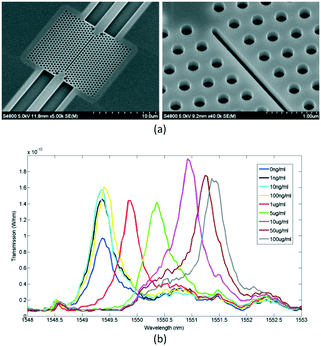 | ||
| Fig. 14 Microscopic structure (a) and sensing property (b) of slot PC cavity for avidin concentration measurement. Adapted from ref. 68, copyright 2011. | ||
Then, in 2012, Chakravarty et al. proposed an L13-typed PC cavity as shown in Fig. 15, in which resonance showed a high-quality factor ∼9300 in the bio-ambient phosphate buffered saline (PBS) and high sensitivity.69 Experimental results demonstrated that an antibody mass sensitivity of 8.8 atto-grams with a sensitivity per unit area of 0.8 pg mm−2 could be achieved.
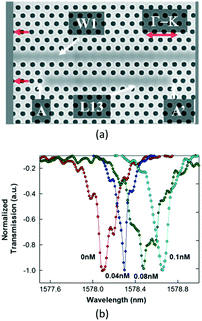 | ||
| Fig. 15 Microscopic structure (a) and sensing property (b) of L13-typed PC cavity for antibody concentration measurement. Adapted from ref. 69, copyright 2012. | ||
Upon this structure, Zou et al. demonstrated that 1 pM (67 pg ml−1) and 50 femto-molar (3.35 pg ml−1) concentrations of avidin binding to biotin in PBS could be detected for the L21 and L55 PC microcavities respectively.70
At the same time, Chakravarty et al. gave detailed analyses of quality factor, fill fraction, and group index of this PC device, to the detection limit for bulk chemical sensing and the minimum detectable biomolecule concentration in biosensing. Slow light in a two-dimensional PC provided the opportunity for significant reduction of the detection limit below 1 × 10−7 RIU, which enabled a highly sensitive sensor in diverse application areas. It was experimentally demonstrated that a concentration of 1 fM (67 fg ml−1) for the binding between biotin and avidin could be detected.71
3.4 On-chip detection of nanoparticle
The ultra-small mode volume of the PC cavity plays an important role in single nanoparticle detection. When a nanoparticle falls in the vicinity of the strongly localized optical field (sensitive region), the resonant wavelength will undergo a gentle shift. Based on the perturbation theory, the resonant shift caused by the nanoparticle is determined by:72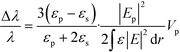 | (2) |
 is the overall optical energy inside the cavity, which is proportional to the mode volume.
is the overall optical energy inside the cavity, which is proportional to the mode volume.
From the equation shown above, a larger wavelength shift is generated as long as the mode volume is smaller on a condition of the same particle size. As previously mentioned, PC cavity can confine the electric field to a very small volume, of the order of a few cubic wavelengths. If a particle is delivered to this high-field region (sensitive region) on the sensor surface, single-particle sensitivity may be achieved. Therefore, there is a significant interest in developing a PC cavity for the ultrasensitive detection of nanoparticles such as viruses.
In 2007, Lee et al. demonstrated that the PC cavity can be used for the detection of a single latex particle that has a diameter of 50 nm.73 The slab-PC consisted of a 685 nm diameter microcavity (see Fig. 16) where a significant amount of electric field was confined. The resonant redshift would increase as the latex sphere diameter increased. A particle that has a diameter of 370 nm would induce a redshift of 4.2 nm. Further, the authors reported that the electric field was concentrated close to the edge of the central hole, which made this region more sensitive to a particle diameter change than the center of the hole.
 | ||
| Fig. 16 Microscopic structure (a) and sensing property (b) of PC cavity for nanoparticle diameter measurement. Adapted from ref. 73, copyright 2007. | ||
Then, in 2010, Baker et al. utilized a defect-free slab-PC design to demonstrate sensitive and size-selective detection of the biological particle.74 In their experiments, the infiltrations of latex particles with diameters of 260 and 320 nm into the PC lattice holes of 280 nm in diameter were investigated. A significant redshift in the band-edge of the PC was observed for the infiltration of smaller particles, thus demonstrating a size-selective particle detection. With this PC structure, it is possible to detect low numbers (<200) of randomly bound virus-sized particles.
In 2013, Descharmes et al. demonstrated a resonant optical trapping mechanism based on a PC cavity (see Fig. 17).75 The PC cavity was implemented in a 30 mm × 12 mm optofluidic chip, which consisted of a patterned silicon substrate and an ultrathin microfluidic membrane for particle injection and control. It was demonstrated that the particle induced a large resonant shift of the cavity mode, amounting to several linewidths. This shift was exploited to detect the presence of a particle within the trap and to retrieve information on the trapped particles.
 | ||
| Fig. 17 Microscopic structure (a) and sensing property (b) of H1-typed PC cavity for nanoparticle detection. Adapted from ref. 75, copyright 2013. | ||
3.5 On-chip detection of cancer cell
Cancer has become one of the leading diseases world-wide, which results in increased morbidity and mortality. Developing a fast, efficient, and accurate method for the detection and diagnosis of cancer in the early stages can contribute to better treatment outcomes and significantly increase the quality of life for cancer patients. The refractive-index ranges of normal cells and cancerous cells are reported as 1.35–1.37 and 1.39–1.401 respectively. Therefore, many PC cavity sensors have been widely employed in the refractive-index detection of cancerous cells.In 2013, Chakravarty et al. proposed a label-free PC cavity biosensor to detect the epithelial-mesenchymal transition (EMT) transcription factor, ZEB1, in lysates from NCI-H358 lung cancer cells.76 By using a L13 PC cavity, an estimated concentration of 2 cells per μL was demonstrated. Multiplexed sensors permit simultaneous detection of many binding interactions with specific immobilized antibodies from the same bio-sample at the same instant of time.
Later, in 2016, an H1-typed PC cavity was proposed for diseased cell detection.77 It was observed that the resonant wavelength of the PC cavity would redshift on increasing the refractive index of the nanocavity imposed by the presence of a cancer cell. This system was able to differentiate a slight change in resonant peak in the transmission spectra when Jurkat and HeLa cell lines were introduced (refractive-index difference of 0.002 RIU).
As for the cell study, it should be mentioned that PC-enhanced microscopy, a new form of optical microscopy that uses a PC surface to dynamically detect and visualize biomaterial-surface interactions, has the ability to make high-resolution images of attached cells, thus enabling us to study cell functions (including cell adhesion, migration, apoptosis, and differentiation).78,79
3.6 Summary and comparison
From the above examples, we can find that with an appropriate choice of receptor to infiltrate the air holes of the PC cavity, a PC cavity sensor could be used for biochemical sensing. The properties of such as system can be further improved by designing PC cavities with higher quality factors and by localizing the target molecular recognition processes in the defect region. The small size of the device, combined with the strong integration of multiple PC cavities, provides a promising potential for large arrays of independent sensors on a centimeter-sized chip. It should be noted that the electromagnetic fields distributions and the sensing properties of the PC cavity are related to the refractive index of the air holes. As different analytes have different refractive indices, the structural design of the PC cavity is not common to all measurement parameters. Specially, for PC-cavity sensors that operate in a liquid environment, the covering of liquid will vastly change the refractive index of the air holes, which will then influence the quality factor and bulk refractive-index sensitivity of the PC-cavity. Therefore, the structural design and fabrication of the PC cavity need to consider the refractive-index range of the target analyte under different concentrations. The aim is to ensure that the PC-cavity sensor can work well with liquid infiltration and exhibit good sensing properties under different liquid concentrations. Table 2 summarizes the structures and corresponding properties of optofluidic PC cavities that are used for biochemical sensing.| Sensing parameter | Structure type | Sensitivity | Detection limit | Quality factor | Ref. |
|---|---|---|---|---|---|
| Gas concentration | Hetero | 80 nm RIU−1 | 1 × 10−5 RIU | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
40 |
| Hetero | 510 nm RIU−1 | 1 × 10−5 RIU | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
41 | |
| Ln-slot | 421 nm RIU−1 | 1 × 10−5 RIU | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
42 | |
| Ring | 363.8 nm RIU−1 | 697.35 ppm | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 923 923 |
43 | |
| Shoulder-coupled | 450 nm RIU−1 | 2.37 ppm | 1105 | 44 | |
| Liquid concentration | Hetero | 1538 nm RIU−1 | 7.8 × 10−6 RIU | 4000 | 47 |
| Half-ring | 293 nm RIU−1 | — | 950 | 48 | |
| L13 | 66 nm RIU−1 | — | 7000 | 49 | |
| Ln | 61.3 nm RIU−1 | 0.006 RIU | 5248 | 51 | |
| Shoulder-coupled | 141.67 nm RIU−1 | — | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
52 | |
| Ring-slot | 160 nm RIU−1 | 8.75 × 10−5 RIU | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 477 477 |
54 | |
| Hm | 115.6 nm RIU−1 | 8.65 × 10−5 RIU | 2761 | 55 | |
| L3 + H1 | 136.67 nm RIU−1 | 6.5 × 10−3 RIU | 2000 | 56 | |
| H0 + H1 | 620 nm RIU−1 | 9.4322 ppm | 2480 | 57 | |
| Protein | H0 | — | 2.5 fg | — | 58 |
| L3 | 24.7 nm pg−1 | 4.0 fg | — | 59 | |
| Point defect | 2.3 × 105 nm M−1 | 1.5 fg | 500 | 60 | |
| Ln | — | 110 pg mm−2 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 760 |
61 | |
| L13 | — | 3.35 pg mL−1 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
62 | |
| DNA | Nano-ring | 0.5 nm fg−1 | 0.2 fg | 3200 | 63 |
| H1 | 1.63 nm fg−1 | 0.061 fg | 4000 | 65 | |
| Nano-ring | 3.4 nm fg−1 | 0.029 fg | 3700 | 66 | |
| Antibiotic | H0 | 176 nm RIU−1 | 20pM | 690 | 67 |
| Slot | — | 15 nM | 3000 | 68 | |
| L13 | — | 0.8 pg mm−2 | 9300 | 69 | |
| L55 | — | 50 fM | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
70 | |
| L55 | — | 1 fM | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
71 | |
| Nanoparticle | Microcavity | — | ≤50 nm | 2000 | 73 |
| Point defect | — | 50 nm | 2000 | 75 | |
| Cancer cell | L13 | — | 2 cells | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 760 |
76 |
| Shoulder-coupled | 388.57 nm RIU−1 | — | 4857 | 77 |
4. Challenges and future research directions
At present, the integration of PC-cavity structures with emerging technologies is promising for the on-chip sensing of biochemical molecules, owing to the compact, flexible, and easy-to-use platforms. However, the field of PC-cavity-based, on-chip biochemical sensors is still at an early stage, and some challenges remain that need to be resolved.4.1 High-precision fabrication and infiltration of PC cavity
The PC cavities presented above are mainly fabricated on a silicon-on-insulator (SOI) substrate by using an electron beam lithography method. Considering the current technology of PC fabrication, the position and size of the critical hole can only be maintained within 1 nm (ref. 80) and 2–4 nm,81 respectively, thus resulting in low reproducibility of these PC structures. However, these fabrication errors have a large influence on the properties of the PC. It was estimated that a 1% fluctuation in hole size would cause the transmission attenuation of 15 dB mm−1.82 Further, to maintain the transmission of a PC with a length of 1 mm above 90%, the position error should be below 0.3% and the size error should be below 0.5%. In particular, the fabrication errors of the cavity region of the PC cavity will have a more significant influence on the resonant properties of the PC cavity, due to the fact that the interaction strength between the optical field and the materials of the defected region is relatively strong. Hagina et al.83 demonstrated that the quality factor of a hetero-typed PC cavity would be reduced to one eighth of the ideal value when only a 1 nm hole size error was introduced. However, due to the particular micro-nano and porous structure of the PC, the fabrication errors of the PC cavity are still complicated, random, unpredictable, and immeasurable. Therefore, simplification of the fabrication complexity and realization of the high-precision fabrication of the PC cavity are major challenges in the future.As for biosensing, which relies on the binding of the target molecule and receptor antibody/protein, the PC-cavity-based sensor can sense only the analytes that land in the high-field region/sensitive region/defected region of PC cavity, but not those that land in all the other places. The vast majority of molecules on the non-sensitive region do not contribute to a wavelength shift of the resonant spectrum. In other words, a biosensor may be sensitive to a single virus or single nanoparticle only if this virus or nanoparticle was landed in the high-field region/sensitive region/defected region of the PC cavity. Therefore, it is necessary to infiltrate the analyte to land on the specific holes of the PC cavity, and not all the other places where the sensor lacks sensitivity. Besides, the sensing properties of the PC cavity are inescapably affected by the precision of the infiltration process. At present, some infiltration methods for PC devices have been proposed, by using an integrated optofluidic circuit that is bonded onto the PC chip84 or lithographic masking,85 a modified confocal laser scanning microscope equipped with a micro-infiltration system,86 a computer-controlled micro-tip,87 or micro-pipette88 whose size is comparable to the air holes to be infiltrated. These technologies are able to achieve local filling of one or more nanometer holes in the specific location of the PC with high precision and good reproducibility. However, during biosensing experiments, after each measurement of a certain concentration of target molecule, the PC device should be washed a number of times before the effective resonant wavelength shift is measured. This will require considerable time, resulting in a low throughput of the PC cavity-based biosensor. Therefore, methods of infiltrating certain air holes of the PC cavity with high precision and high speed is also a major challenge in the future work.
4.2 Integrating sensing platform
Most biochemical sensors based on a PC cavity operate as a point or single mode, whose throughputs are low due to the necessity to measure each detected region separately with an individual detector, and thus also increase the sample volume needed for measurement. To overcome these drawbacks, an ideal method is to detect multiple targets in one integrated biochemical sensing platform. This feature will provide a wide window to comprehensively evaluate many biomolecules, such as proteins, DNA molecules, small molecules, or virus particles. For this reason, many integrated sensing platforms based on cascaded PC cavities have been developed.60,78–85In 2011, Pal et al.60 demonstrated an integrated lgG sensor, in which three PC cavities were cascaded in series. However, the sensor volume was too large and not suitable for sensing applications. Then, in 2012, Wang et al.89 built a theoretical model of an integrated parallel self-collimation sensor array. However, only three sensors could be integrated on the monolithic platform, resulting in a low integration density. At the same time, Yang et al.90 theoretically investigated the integrated properties of the H0-typed PC cavity for multi-point refractive-index sensing. Then, in 2014, Yang et al.91 designed a PC beam-splitter, which consisted of parallel output waveguides. It was demonstrated that some H0-typed PC cavities could be side-coupled to any waveguides, and allowed the implementation of a simple but functional sensor array. At the same time, Liu et al.56 proposed a radius-graded PC sensor array, where two L3 cavities and two H1 cavities were multiplexed and interlaced on both sides of a PC waveguide. In addition, Olyaee et al.65 have demonstrated that the sensor array could also be realized when some H1-typed PC cavities were side-coupled to a PC waveguide. Later, in 2016, to enhance the measurement sensitivity, the integrated slot the PC cavity was introduced (see Fig. 18) by Fu et al.92 and Yang et al.93
 | ||
| Fig. 18 Schematic structure of integrated slot PC cavity.92 | ||
However, the main drawback of these side-coupled resonant cavity arrays was that the greater the number of PC cavities integrated on the monolithic platform, the narrower the spacing of the adjacent resonant peaks. For sensing application, the resonant peak might cross with each other if the variation of one resonant signal caused by the target parameter change is too large, which will result in difficulties in recognizing the sensing signals from different cavities.
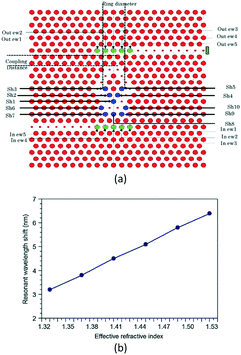
To effectively increase the integrated density of the sensor array and avoid crosstalk of adjacent PC cavities, Huang et al.94 proposed a low crosstalk ring-slot array structure for label-free multi-parameter sensing. The structure was based on an array of three ring-slot and input/output line defect coupling waveguides (see Fig. 19(a)). Each ring-slot cavity had a slightly different cavity spacing and a different resonant frequency. Then, in 2016, to further enhance the sensitivity of each sensor, Zhou et al.95 proposed a new integrated structure using a well-designed 1 × 3 PC waveguide splitter and an elaborate single-slot PC cavity (see Fig. 19(b)).
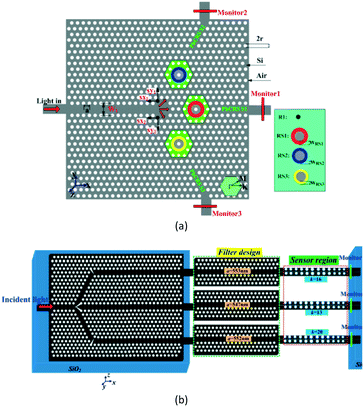 | ||
| Fig. 19 Schematic structure of integrated PC cavity with (a) three ring-slot and (b) PC waveguide splitter. Adapted from ref. 94, copyright 2014, and ref. 95, copyright 2016. | ||
Although the above cascaded PC cavities can improve the integration degree of PC-cavity-based sensor and then achieve simultaneous multi-element detection, the surface of each PC cavity in the array should be immobilized with a specific binding material to detect the corresponding element in the mixture, which severely limits the sensing flexibility and requires massive external equipment and complex device packaging. In comparison with the normally-illuminated PC biosensors that have a very high degree of multiplex,11,16 the multiplexing throughput of the PC-cavity-based biosensors should also be improved. Recently, Zhang et al.96 proposed another integrated scheme, by using the real and imaginary parts of the refractive index. Experimental results demonstrated that both the resonant wavelength and linewidth changed linearly with the real and imaginary parts of the refractive index, respectively. Therefore, two mutually independent physical quantities were obtained during the detection, and the concentration composition of a ternary mixture, which had two unknown parameters, could be determined without any surface immobilizations; thus providing an effective solution to resolve the limitations in multiplexing throughput of PC-cavity-based biosensors.
4.3 Response time
A PC-cavity-based biosensor can detect a single biomolecule, but it should be mentioned that the diffusion velocity of a biomolecule from microfluidic will limit the minimum detectable analyte concentration.97 For ease of measurement and device portability, a common method is to deliver the measured sample to the biosensor by using on-chip streams of fluid, which is driven by pressure or electrokinesis.68,96 The microfluidic channel layer that is placed on the top of the PC layer can be fabricated by soft lithography using a silicon template, and then bonded onto the PC chip after undergoing an oxygen plasma treatment. Then, in the detection process, molecules flowing above the sensor will diffuse down to the sensor surface, near the PC cavity. However, the detection should also take a few minutes in terms of practical measurement. In particular, in some filtration-typed sample preparation steps, it is required to concentrate targets in the sample, which also increases the device complexity and detection time. Considering the practical application perspective, biochemical sensors need to be fabricated with inexpensive materials and simple production techniques. Therefore, it is necessary to provide rapid, inexpensive, and multiplexed infiltration solutions.4.4 Novel smart sensitive materials
In a biochemical sensor, the introduction of smart sensitive materials, such as hydrogels, polyionic liquids, graphene, and carbon nanotubes, is significant due to the fact that their physical or chemical properties will be modified along with surrounding responsive substances.98–101 In particular, their incorporation with PC structures holds considerable promise for rapid, sensitive, and reliable biochemical sensing.102–106 For instance, PC structures comprised of hydrogel materials can be used as biosensors for detection of DNA, proteins, antibodies, and enzymes by monitoring the changes in lattice spacing or refractive index.102,103 In this respect, hydrogel-based PC structures provide quantitative spectral results of target biomolecule concentrations. Taking advantage of flexible (e.g., hydrogels) and smart (e.g., carbon nanotubes and graphene) materials, PC-based sensors will be an alternative to the current wearable continuous monitoring tools and sensors.1075. Conclusions
In this paper, the most typical on-chip biochemical sensors based on optofluidic PC cavities over recent years were reported, with specific focuses on their structures, development processes, sensing principles, advantages, and disadvantages. Besides, the existing challenges and future research directions were noted. The review of these reported works and their corresponding results demonstrates that PC cavities combined with optofluidic technologies have played very important roles in the biochemical sensing fields and will produce a significant industrial value. Clearly, readers who are interested in this field can not only see the special properties and flexibilities in the structural design of optofluidic PC cavity, but can also broaden their thoughts and innovate with new solutions to further exploit more novel biochemical sensors. In addition, more design schemes involving optofluidic PC cavities will be proposed and better on-chip biochemical sensing properties will be presented, along with technology developments of PC fabrication and microfluidic infiltration, which will then inspire a promising future for PC-based sensors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported in part by the National Science Foundation for Distinguished Young Scholars of China under Grant 61425003, the National Natural Science Foundation of China under Grant 61703080, 61273059 and 61371200, the Fundamental Research Funds for the Central Universities under Grant N160404012 and N150401001, the Liaoning Province Natural Science Foundation under Grant 20170540314, and State Key Laboratory of Synthetical Automation for Process Industries under Grant 2013ZCX09.References
- P. Mehrotra, Biosensors and their applications - A review, J. Oral Biol. Craniofac. Res., 2016, 6, 153–159 CrossRef PubMed.
- C. Ciminellin, C. M. Campanella, F. Dell'Olio, C. E. Campanella and M. N. Armenise, Label-free optical resonant sensors for biochemical applications, Prog. Quantum. Electron., 2013, 37, 51–107 CrossRef.
- G. Zanchetta, R. Lanfranco, F. Giavazzi, T. Bellini and M. Buscaglia, Emerging applications of label-free optical biosensors, Nanophotonics, 2017, 6(4), 627–645 CrossRef CAS.
- J. Homola, Surface plasmon resonance sensors for detection of chemical and biological species, Chem. Rev., 2008, 108(2), 462–493 CrossRef CAS PubMed.
- E. Kim, M. D. Baaskea and F. Vollmer, Towards next-generation label-free biosensors: recent advances in whispering gallery mode sensors, Lab Chip, 2017, 17, 1190–1205 RSC.
- J. Su, Label-free biological and chemical sensing using whispering gallery mode optical resonators: past, present, and future, Sensors, 2017, 17, 540 CrossRef PubMed.
- M. Baaske and F. Vollmer, Optical resonator biosensors: molecular diagnostic and nanoparticle detection on an integrated platform, ChemPhysChem, 2012, 13(2), 427–436 CrossRef CAS PubMed.
- F. Chiavaioli, F. Baldini, S. Tombelli, C. Trono and A. Giannetti, Biosensing with optical fiber gratings, Nanophotonics, 2017, 6(4), 663–679 CrossRef CAS.
- N. Descharmes, U. P. Dharanipathy, Z. Diao, M. Tonin and R. Houdre, Single particle detection manipulation and analysis with resonant optical trapping in photonic crystals, Lab Chip, 2013, 13(16), 3268–3274 RSC.
- Y. Zou, S. Chakravarty, W. C. Lai, C. Y. Lin and R. T. Chenab, Methods to array photonic crystal microcavities for high throughput high sensitivity biosensing on a silicon-chip based platform, Lab Chip, 2012, 12(13), 2309–2312 RSC.
- J. E. Baker, R. Sriram and B. L. Miller, Two-dimensional photonic crystals for sensitive microscale chemical and biochemical sensing, Lab Chip, 2015, 15(4), 971–990 RSC.
- H. Inan, M. Poyraz, F. Inci, M. A. Lifson, M. Baday, B. T. Cunningham and U. Demirci, Photonic crystals: emerging biosensors and their promise for point-of-care applications, Chem. Soc. Rev., 2017, 46(2), 366–388 RSC.
- C. Ge, M. Lu, S. George, T. A. Flood, C. Wagner, J. Zheng, A. Pokhriyal, J. G. Eden, P. J. Hergenrotherd and B. T. Cunningham, External cavity laser biosensor, Lab Chip, 2013, 13, 1247–1256 RSC.
- C. Fenzl, T. Hirsch and O. S. Wolfbeis, Photonic crystals for chemical sensing and biosensing, Angew. Chem., Int. Ed., 2014, 53(13), 3318–3335 CrossRef CAS PubMed.
- Q. Huang, J. Peh, P. J. Hergenrother and B. T. Cunningham, Porous photonic crystal external cavity laser biosensor, Appl. Phys. Lett., 2016, 109, 071103 CrossRef PubMed.
- C. Ge, External cavity laser label-free biosensor, University of Illinois at Urbana-Champaign, 2013 Search PubMed.
- M. Llera, T. Aellen, J. Hervas, Y. Salvadé, P. Senn, S. Le Floch and H. Keppner, Liquid-air based Fabry-Pérot cavity on fiber tip sensor, Opt. Express, 2016, 24(8), 8054–8065 CrossRef CAS PubMed.
- H. Yan, Y. Zou, S. Chakravarty, C. J. Yang, Z. Wang, N. M. Tang, D. L. Fan and R. T. Chen, Silicon on-chip bandpass filters for the multiplexing of high sensitivity photonic crystal microcavity biosensors, Appl. Phys. Lett., 2015, 106(12), 121103 CrossRef PubMed.
- Y. G. Zhang, S. Han, S. B. Zhang, S. L. Zhang, P. H. Liu and Y. C. Shi, High-Q, high-sensitivity photonic crystal cavity sensor, IEEE Photonics J., 2015, 7(5), 6802906 Search PubMed.
- M. Aas, Q. S. Chen, A. Jonas, A. Kiraz and X. D. Fan, Optofluidic FRET lasers and their applications in novel photonic devices and biochemical sensing, IEEE J. Sel. Top. Quantum Electron., 2016, 22(4), 7000215 CrossRef.
- D. Psaltis, S. Quake and C. Yang, Developing optofluidic technology through the fusion of microfluidics and optics, Nature, 2006, 442, 381–386 CrossRef CAS PubMed.
- N. W. L. Speijcken, M. A. Dündar, A. C. Bedoya, C. Monat, C. Grillet, P. Domachuk, R. Nötzel, B. J. Eggleton and R. W. van der Heijden, In situ optofluidic control of reconfigurable photonic crystal cavities, Appl. Phys. Lett., 2012, 100(26), 261107 CrossRef.
- S. Feng, J. H. Jiang, A. A. Rashid and S. John, Biosensor architecture for enhanced disease diagnostics: lab-in-a-photonic-crystal, Opt. Express, 2016, 24(11), 12166–12191 CrossRef CAS PubMed.
- J. D. Joannopoulos, S. G. Johnson, J. N. Winn and R. D. Meade, Photonic crystals: Molding the flow of light (Second Edition), Princeton University Press, 2008 Search PubMed.
- R. V. Nair and R. Vijaya, Photonic crystal sensors: An overview, Prog. Quantum Electron., 2010, 34(3), 89–134 CrossRef CAS.
- N. A. Mortensen, S. S. Xiao and J. Pedersen, Liquid-infiltrated photonic crystals: enhanced light-matter interactions for lab-on-a-chip applications, Microfluid. Nanofluid., 2008, 4, 117–127 CrossRef CAS.
- Y. Zou, S. Chakravarty, D. N. Kwong, W. C. Lai, X. C. Xu, X. H. Lin, A. Hosseini and R. T. Chen, Cavity-waveguide coupling engineered high sensitivity silicon photonic crystal microcavity biosensors with high yield, IEEE J. Sel. Top. Quantum Electron., 2014, 20(4), 6900710 Search PubMed.
- H. Sekoguchi, Y. Takahashi, T. Asano and S. Noda, Photonic crystal nanocavity with a Q-factor of ~9 million, Opt. Express, 2014, 22(1), 916–924 CrossRef PubMed.
- D. Yang, H. Tian and Y. Ji, The properties of lattice-shifted microcavity in photonic crystal slab and its applications for electro-optical sensor, Sens. Actuators, A, 2011, 171(2), 146–151 CrossRef CAS.
- Y. N. Zhang, Y. Zhao, D. Wu and Q. Wang, Fiber loop ring-down refractive index sensor based on high-Q photonic crystal cavity, IEEE Sens. J., 2014, 14(6), 1878–1885 CrossRef.
- Y. Zhang, D. P. Li, C. Zeng, X. Z. Qiu, G. Gao, Y. Wang, Q. Z. Huang, J. Z. Yu and J. S. Xia, Low power and large modulation depth optical bistability in an Si photonic crystal L3 cavity, IEEE Photonics Technol. Lett., 2014, 26(23), 2399–2402 CrossRef.
- Y. Yang, D. Q. Yang, H. P. Tian and Y. F. Ji, Photonic crystal stress sensor with high sensitivity in double directions based on shoulder-coupled aslant nanocavity, Sens. Actuators, A, 2013, 193, 149–154 CrossRef CAS.
- A. Majumdar, J. Kim, J. Vuckovic and F. Wang, Electrical control of silicon photonic crystal cavity by grapheme, Nano Lett., 2013, 13(2), 515–518 CrossRef CAS PubMed.
- B. Li and C. Lee, NEMS diaphragm sensors integrated with triple-nano-ring resonator, Sens. Actuators, A, 2011, 172(1), 61–68 CrossRef CAS.
- T. M. Trong, F. L. Hsiao, C. Lee, W. F. Xiang, C. C. Chen and W. K. Choi, Optimization and comparison of photonic crystal resonators for silicon microcantilever sensors, Sens. Actuators, A, 2011, 165(1), 16–25 CrossRef.
- C. Caer, S. F. Serna-Otálvaro, W. Zhang, X. Le Roux and E. Cassan, Liquid sensor based on high-Q slot photonic crystal cavity in silicon-on-insulator configuration, Opt. Lett., 2014, 39(20), 5792–5794 CrossRef PubMed.
- J. H. Wülbern, J. Hampe, A. Petrov, M. Eich, J. D. Luo, A. Jen, A. Di Falco, T. F. Krauss and J. Bruns, Electro-optic modulation in slotted resonant photonic crystal heterostructures, Appl. Phys. Lett., 2009, 94(24), 241107 CrossRef.
- Y. Zou, S. Chakravarty, L. Zhu and R. T. Chen, The role of group index engineering in series-connected photonic crystal microcavities for high density sensor microarrays, Appl. Phys. Lett., 2014, 104, 141103 CrossRef PubMed.
- T. V. Dinh, I. Y. Choi, Y. S. Son and J. C. Kim, A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction, Sens. Actuators, B, 2016, 231, 529–538 CrossRef CAS.
- T. Sünner, T. Stichel, S. H. Kwon, T. W. Schlereth, S. Hofling, M. Kamp and A. Forchel, Photonic crystal cavity based gas sensor, Appl. Phys. Lett., 2008, 92(26), 261112 CrossRef.
- J. Jágerská, H. Zhang, Z. L. Diao, N. Le Thomas and R. Houdre, Refractive index sensing with an air-slot photonic crystal nanocavity, Opt. Lett., 2010, 35(15), 2523–2525 CrossRef PubMed.
- K. Z. Li, J. T. Li, Y. J. Song, G. S. Fang, C. Li, Z. Y. Feng, R. B. Su, B. H. Zeng, X. H. Wang and C. J. Jin, L-n slot photonic crystal microcavity for refractive index gas sensing, IEEE Photonics J., 2014, 6(5), 6802509 Search PubMed.
- Y. Zhang, Y. Zhao and Q. Wang, Measurement of methane concentration with cryptophane E infiltrated photonic crystal microcavity, Sens. Actuators, B, 2015, 209, 431–437 CrossRef CAS.
- X. L. Qian, Y. Zhao, Y. N. Zhang and Q. Wang, Theoretical research of gas sensing method based on photonic crystal cavity and fiber loop ring-down technique, Sens. Actuators, B, 2016, 228, 665–672 CrossRef CAS.
- J. Hodgkinson and R. P. Tatam, Optical gas sensing: a review, Meas. Sci. Technol., 2013, 24, 012004 CrossRef.
- A. C. Liapis, B. S. Gao, M. R. Siddiqui, Z. M. Shi and R. W. Boyd, On-chip spectroscopy with thermally tuned high-Q photonic crystal cavities, Appl. Phys. Lett., 2016, 108(2), 021105 CrossRef.
- A. Di Falco, L. O'Faolain and T. F. Krauss, Chemical sensing in slotted photonic crystal heterostructure cavities, Appl. Phys. Lett., 2009, 94(6), 063503 CrossRef.
- F. Hosseinibalam, S. Hassanzadeh, A. Ebnali-Heidari and C. Karnutsch, Design of an optofluidic biosensor using the slow-light effect in photonic crystal structures, Appl. Opt., 2012, 51(05), 568–576 CrossRef CAS PubMed.
- W. C. Lai, S. Chakravarty, Y. Zou, Y. B. Guo and R. T. Chen, Slow light enhanced sensitivity of resonance modes in photonic crystal biosensors, Appl. Phys. Lett., 2013, 102(4), 041111 CrossRef PubMed.
- C. P. Ho, B. Li, A. J. Danner and C. Lee, Design and modeling of 2-D photonic crystals based hexagonal triple-nano-ring resonators as biosensors, Microsyst. Technol., 2013, 19(1), 53–60 CrossRef CAS.
- S. Olyaee and S. Najafgholinezhad, A high quality factor and wide measurement range biosensor based on photonic crystal nanocavity resonator, Sens. Lett., 2013, 11(3), 483–488 CrossRef CAS.
- S. Najafgholinezhad and S. Olyaee, A photonic crystal biosensor with temperature dependency investigation of micro-cavity resonator, Optik, 2014, 125(21), 6562–6565 CrossRef.
- J. Zhou, H. P. Tian, D. Q. Yang, Q. Liu and Y. F. Ji, Integration of high transmittance photonic crystal H2 nanocavity and broadband W1 waveguide for biosensing applications based on Silicon-on-Insulator substrate, Opt. Commun., 2014, 330, 175–183 CrossRef CAS.
- L. J. Huang, H. P. Tian, J. Zhou, Q. Liu, P. Zhang and Y. F. Ji, Label-free optical sensor by designing a high-Q photonic crystal ring-slot structure, Opt. Commun., 2015, 335, 73–77 CrossRef CAS.
- D. Q. Yang, H. P. Tian and Y. F. Ji, Nanoscale photonic crystal sensor arrays on monolithic substrates using side-coupled resonant cavity arrays, Opt. Express, 2011, 19(21), 20023–20034 CrossRef PubMed.
- Q. Liu, H. P. Tian, D. Q. Yang, J. Zhou, Y. Yang and Y. F. Ji, Nanoscale radius-graded photonic crystal sensor arrays using interlaced and symmetrical resonant cavities for biosensing, Sens. Actuators, A, 2014, 216, 223–230 CrossRef CAS.
- Y. N. Zhang, Y. Zhao and H. F. Hu, Miniature photonic crystal cavity sensor for simultaneous measurement of liquid concentration and temperature, Sens. Actuators, B, 2015, 216, 563–571 CrossRef CAS.
- M. Lee and P. M. Fauchet, Two-dimensional silicon photonic crystal based biosensing platform for protein detection, Opt. Express, 2007, 15(8), 4530–4535 CrossRef CAS PubMed.
- D. Dorfner, T. Zabel, T. Hurlimann, N. Hauke, L. Frandsen, U. Rant, G. Abstreiter and J. Finley, Photonic crystal nanostructures for optical biosensing applications, Biosens. Bioelectron., 2009, 24(12), 3688–3692 CrossRef CAS PubMed.
- S. Pal, E. Guillermain, R. Sriram, B. L. Miller and P. M. Fauchet, Silicon photonic crystal nanocavity-coupled waveguides for error-corrected optical biosensing, Biosens. Bioelectron., 2011, 26(10), 4024–4031 CrossRef CAS PubMed.
- W. C. Lai, S. Chakravarty, Y. Zou and R. T. Chen, Silicon nano-membrane based photonic crystal microcavities for high sensitivity bio-sensing, Opt. Lett., 2012, 37(7), 1208–1210 CrossRef CAS PubMed.
- C. J. Yang, N. M. Tang, H. Yan, S. Chakravarty, D. H. Li and R. T. Chen, 193 nm lithography fabricated high sensitivity photonic crystal microcavity biosensors for plasma protein detection in patients with pancreatic cancer, 2015 Conference on Lasers and Electro-Optics, San Jose, CA, USA, 13 August 2015, p. 15365354 Search PubMed.
- F. L. Hsiao and C. Lee, Computational study of photonic crystals nano-ring resonator for biochemical sensing, IEEE Sens. J., 2010, 10(7), 1185–1191 CrossRef CAS.
- F. L. Hsiao and C. K. Lee, Nanophotonic biosensors using hexagonal nanoring resonators: computational study, J. Micro. Nanolithogr. MEMS MOEMS, 2011, 10(1), 013001 CrossRef.
- S. Olyaee and S. Najafgholinezhad, Computational study of a label-free biosensor based on a photonic crystal nanocavity resonator, Appl. Opt., 2013, 52(29), 7206–7213 CrossRef CAS PubMed.
- S. Olyaee and A. M. Bahabady, Design and optimization of diamond-shaped biosensor using photonic crystal nano-ring resonator, Optik, 2015, 126(20), 2560–2564 CrossRef CAS.
- S. Zlatanovic, L. W. Mirkarimi, M. M. Sigalas, M. A. Bynum, E. Chow, K. M. Robotti, G. W. Burr, S. Esener and A. Grot, Photonic crystal microcavity sensor for ultracompact monitoring of reaction kinetics and protein concentration, Sens. Actuators, B, 2009, 141(1), 13–19 CrossRef CAS.
- M. G. Scullion, A. Di Falco and T. F. Krauss, Slotted photonic crystal cavities with integrated microfluidics for biosensing applications, Biosens. Bioelectron., 2011, 27(1), 101–105 CrossRef CAS PubMed.
- S. Chakravarty, Y. Zou, W. C. Lai and R. T. Chen, Slow light engineering for high Q high sensitivity photonic crystal microcavity biosensors in silicon, Biosens. Bioelectron., 2012, 38(1), 170–176 CrossRef CAS PubMed.
- Y. Zou, S. Chakravarty, D. N. Kwong, W. C. Lai, X. C. Xu, X. H. Lin, A. Hosseinin and R. T. Chen, Cavity-waveguide coupling engineered high sensitivity silicon photonic crystal microcavity biosensors with high yield, IEEE J. Sel. Top. Quantum. Electron., 2014, 20(4), 900710 Search PubMed.
- S. Chakravarty, A. Hosseini, X. C. Xu, L. Zhu, Y. Zou and R. T. Chen, Analysis of ultra-high sensitivity configuration in chip-integrated photonic crystal microcavity biosensors, Appl. Phys. Lett., 2014, 104(19), 191109 CrossRef PubMed.
- T. Lin, X. W. Zhang, G. Y. Zhou, C. F. Siong and J. Deng, Design of an ultra-compact slotted photonic crystal nanobeam cavity for biosensing, J. Opt. Soc. Am. B, 2015, 32(9), 1788–1791 CrossRef CAS.
- M. R. Lee and P. M. Fauchet, Nanoscale microcavity sensor for single particle detection, Opt. Lett., 2007, 32(22), 3284–3286 CrossRef PubMed.
- S. E. Baker, M. D. Pocha, A. S. P. Chang, D. J. Sirbuly, S. Cabrini, S. D. Dhuey, T. C. Bond and S. E. Letant, Detection of bio-organism simulants using random binding on a defect-free photonic crystal, Appl. Phys. Lett., 2010, 97(11), 113701 CrossRef.
- N. Descharmes, U. P. Dharanipathy, Z. Diao, M. Tonin and R. Houdre, Single particle detection, manipulation and analysis with resonant optical trapping in photonic crystals, Lab Chip, 2013, 13(16), 3268–3274 RSC.
- S. Chakravarty, W. C. Lai, Y. Zou, H. A. Drabkin, R. M. Gemmill, G. R. Simon, S. H. Chin and R. T. Chen, Multiplexed specific label-free detection of NCI-H358 lung cancer cell line lysates with silicon based photonic crystal microcavity biosensors, Biosens. Bioelectron., 2013, 43, 50–55 CrossRef CAS PubMed.
- S. Jindal, S. Sobti, M. Kumar, S. Sharma and M. K. Pal, Nanocavity-coupled photonic crystal waveguide as highly sensitive platform for cancer detection, IEEE Sens. J., 2016, 16(10), 3705–3710 CrossRef.
- W. L. Chen, K. D. Long, M. Lu, V. Chaudhery, H. Yu, J. S. Choi, J. Polans, Y. Zhuo, B. A. C. Harley and B. T. Cunningham, Photonic crystal enhanced microscopy for imaging of live cell adhesion, Analyst, 2013, 138(20), 5886–5894 RSC.
- Y. Zhuo and B. T. Cunningham, Label-free biosensor Imaging on Photonic Crystal Surfaces, Sensors, 2015, 15, 21613–21635 CrossRef CAS PubMed.
- L. O'Faolain, T. P. White, D. O'Brien, X. D. Yuan, M. D. Settle and T. F. Krauss, Dependence of extrinsic loss on group velocity in photonic crystal waveguides, Opt. Express, 2007, 15(20), 13129–13138 CrossRef.
- D. M. Beggs, L. O'Faolain and T. F. Krauss, Accurate determination of the functional hole size in photonic crystal slabs using optical methods, Photonics Nanostruct., 2008, 6(3–4), 213–218 CrossRef.
- D. Pergande, T. M. Geppert, A. von Rhein, S. L. Schweizer, R. B. Wehrspohn, S. Moretton and A. Lambrecht, Miniature infrared gas sensors using photonic crystals, J. Appl. Phys., 2011, 109(8), 083117 CrossRef.
- H. Hagina, Y. Takahashi, Y. Tanaka, T. Asano and S. Noda, Effects of fluctuation in air hole radii and positions on optical characteristics in photonic crystal heterostructure nanocavities, Phys. Rev. B, 2009, 79(8), 085112 CrossRef.
- S. H. Kim, J. H. Choi, S. K. Lee, S. H. Kim, S. M. Yang, Y. H. Lee, C. Seassal, P. Regrency and P. Viktorovitch, Optofluidic integration of a photonic crystal nanolaser, Opt. Express, 2008, 16(9), 6515–6527 CrossRef PubMed.
- H. H. J. E. Kicken, P. F. A. Alkemade, R. W. van der Heijden, F. Karouta, R. Notzel, E. van der Drift and H. W. M. Salemink, Wavelength tuning of planar photonic crystals by local processing of individual holes, Opt. Express, 2009, 17(24), 22005–22011 CrossRef CAS PubMed.
- F. Intonti, S. Vignolini, V. Turck, M. Colocci, P. Bettotti, L. Pavesi, S. L. Schweizer, R. Wehrspohn and D. Wiersma, Rewritable photonic circuits, Appl. Phys. Lett., 2006, 89(21), 211117 CrossRef.
- U. Bog, C. L. C. Smith, M. W. Lee, S. Tomljenovic-Hanic, C. Grillet, C. Monat, L. O'Faolain, C. Karnutsch, T. F. Krauss and R. C. McPhedran, High-Q microfluidic cavities in silicon-based two-dimensional photonic crystal structures, Opt. Lett., 2008, 33(19), 2206–2208 CrossRef PubMed.
- C. L. C. Smith, D. K. C. Wu and M. W. Lee, et al., Microfluidic photonic crystal double heterostructures, Appl. Phys. Lett., 2007, 91, 121103 CrossRef.
- Y. F. Wang, H. L. Wang, Q. K. Xue and W. H. Zheng, Photonic crystal self-collimation sensor, Opt. Express, 2012, 20(11), 12111–12118 CrossRef PubMed.
- D. Q. Yang, H. P. Tian, N. N. Wu, Y. Yang and Y. F. Ji, Nanoscale torsion-free photonic crystal pressure sensor with ultra-high sensitivity based on side-coupled piston-type microcavity, Sens. Actuators, A, 2013, 199, 30–36 CrossRef CAS.
- D. Q. Yang, H. P. Tian and Y. F. Ji, Nanoscale low crosstalk photonic crystal integrated sensor array, IEEE Photonics J., 2014, 6(1), 4200107 Search PubMed.
- Z. Y. Fu, J. Zhou, L. J. Huang, F. J. Sun and H. P. Tian, Performance investigation of side-coupled interlaced symmetric-shaft-shape photonic crystal sensor arrays, Opt. Commun., 2016, 381, 146–151 CrossRef CAS.
- D. Q. Yang, C. H. Wang, W. Yuan, B. Wang, Y. J. Yang and Y. F. Ji, Silicon on-chip side-coupled high-Q micro-cavities for the multiplexing of high sensitivity photonic crystal integrated sensors array, Opt. Commun., 2016, 374, 1–7 CrossRef CAS.
- L. J. Huang, H. P. Tian, J. Zhou and Y. F. Ji, Design low crosstalk ring-slot array structure for label-free multiplexed sensing, Sensors, 2014, 14(9), 15658–15668 CrossRef PubMed.
- J. Zhou, L. J. Huang, Z. Y. Fu, F. J. Sun and H. P. Tian, Multiplexed simultaneous high sensitivity sensors with high-order mode based on the integration of photonic crystal 1×3 beam splitter and three different single-slot PCNCs, Sensors, 2016, 16(7), 1050 CrossRef PubMed.
- X. W. Zhang, G. Y. Zhou, P. Shi, H. Du, T. lin, J. H. Teng and F. S. Chau, On-chip integrated optofluidic complex refractive index sensing using silicon photonic crystal nanobeam cavities, Opt. Lett., 2016, 41(6), 1197–1200 CrossRef PubMed.
- P. E. Sheehan and L. J. Whitman, Detection limits for nanoscale biosensors, Nano Lett., 2005, 5(4), 803–807 CrossRef CAS PubMed.
- E. Mastronardi, A. Foster, X. R. Zhang and M. C. DeRosa, Smart materials based on DNA aptamers: taking aptasensing to the next level, Sensors, 2014, 14(2), 3156–3171 CrossRef PubMed.
- R. Verma, R. R. Adhikary and R. Banerjee, Smart material platforms for miniaturized devices: implications in disease models and diagnostics, Lab Chip, 2016, 16(11), 1978–1992 RSC.
- Y. Songa, Y. N. Luoa, C. Z. Zhu, H. Li, D. Du and Y. H. Lin, Recent advances in electrochemical biosensors based on graphene two-dimensional nanomaterials, Biosens. Bioelectron., 2016, 76, 195–212 CrossRef PubMed.
- N. Yang, X. P. Chen, T. L. Ren, P. Zhang and D. G. Yang, Carbon nanotube based biosensors, Sens. Actuators, B, 2015, 207, 690–715 CrossRef CAS.
- Z. Y. Cai, L. A. Luck, D. Punihaole, J. D. Madurac and S. A. Asher, Photonic crystal protein hydrogel sensor materials enabled by conformationally induced volume phase transition, Chem. Sci., 2016, 7(7), 4557–4562 RSC.
- Z. Y. Cai, J. T. Zhang, F. Xue, Z. M. Hong, D. Punihaole and S. A. Asher, 2D photonic crystal protein hydrogel coulometer for sensing serum albumin ligand binding, Anal. Chem., 2014, 86(10), 4840–4847 CrossRef CAS PubMed.
- J. Y. Xu, C. X. Yan, C. Liu, C. H. Zhou, X. C. Hu and F. L. Qi, Photonic crystal hydrogel sensor for detection of nerve agent, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 167, 012024 CrossRef.
- Y. Chen, J. Dong, T. Liu, Q. G. Zhu and W. G. Chen, Refractive index sensing performance analysis of photonic crystal containing graphene based on optical Tamm state, Mod. Phys. Lett. B, 2016, 30(4), 1650030 CrossRef CAS.
- S. M. Zhu, T. Lu and D. Zhang, Progress in stimuli-responsive photonic crystals with biological structures, Acta Polym. Sin., 2017, 2, 229–244 Search PubMed.
- A. Tricoli, N. Nasiri and S. Y. De, Wearable and miniaturized sensor technologies for personalized and preventive medicine, Adv. Funct. Mater., 2017, 27(15), 1605271 CrossRef.
| This journal is © The Royal Society of Chemistry 2018 |




