Open Access Article
Open Access ArticleA novel wireless paper-based potentiometric platform for monitoring glucose in blood†
Rocío
Cánovas
 ,
Marc
Parrilla
,
Marc
Parrilla
 ,
Pascal
Blondeau
,
Pascal
Blondeau
 and
Francisco J.
Andrade
and
Francisco J.
Andrade
 *
*
Department of Analytical and Organic Chemistry, Universitat Rovira i Virgili, 43007, Tarragona, Spain. E-mail: franciscojavier.andrade@urv.cat
First published on 14th June 2017
Abstract
A novel low-cost, compact and sensitive paper-based platform for the accurate monitoring of glucose in biological fluids is presented. Paper-based working and reference electrodes are combined to build a whole potentiometric cell, which also fits a sampling module for simple and fast determination of glucose in a single drop of blood. The working electrode is built using a platinized filter paper coated with a Nafion membrane that entraps the enzyme glucose oxidase; the reference electrode is made by casting a polyvinylbutyral-based membrane onto a conductive paper. The system works by detecting the hydrogen peroxide generated as a result of the enzymatic reaction. Selectivity is achieved due to the permselective behaviour of Nafion, while a significant enhancement of the sensitivity is reached by exploiting the Donnan-coupled formal potential. Under optimum conditions, a sensitivity of −95.9 ± 4.8 mV per decade in the 0.3–3 mM range is obtained. Validation of the measurements has been performed against standard methods in human serum and blood. Final integration with a wireless reader allows for truly in situ measurements with a less than 2 minute procedure including a two-point calibration, washing and measurement. This low-cost analytical device opens up new prospects for rapid diagnostic results in non-laboratory settings.
Introduction
The sharp rise in the number of people affected by chronic diseases is creating a growing problem that largely exceeds the capacities of current healthcare models and infrastructures. For this reason, there is a general agreement that approaches where patients can be more involved in the self-management of their conditions are the best way to face this challenge. During the 21st century, the use of home-based devices for monitoring clinical parameters has become a common way to enhance the quality of life of the patients and improve healthcare systems,1,2 also providing access to those who cannot be easily reached by traditional solutions. Nevertheless, developing home-based instruments at the mass-market level is a paradigm-shifting challenge for analytical science, since factors such as the simplicity of operation and affordability become as relevant as the robustness of the information generated.3,4 Only by addressing these three factors simultaneously, namely: analytical performance, decentralized usability and cost, can these future devices be successfully adopted at a massive scale.5,6The monitoring of blood glucose levels is a good example of challenges that are still unsolved.7,8 The significant improvements brought by the home glucometer9 cannot still be accessed by a large number of patients,10 and this problem is expected to worsen in the near future. Estimations show that by 2030 more than 430 million people – most of them from developing countries – will be affected by diabetes.11,12 Thus, significant efforts towards the development of highly affordable approaches,13,14 ranging from new sensors to alternative detection schemes, are increasingly needed.
The use of paper as a substrate to build analytical tools has emerged during the last few decades as one of the most promising solutions to produce simple, powerful and low-cost analytical platforms.15–17 The group of Whitesides pioneered the development of microfluidic devices exploiting the capillarity of cellulose fibres to perform complex analytical operations18,19 using both colorimetric and electrochemical detection.20–22 Paper-based amperometric sensors for the determination of metals in water23 and for the evaluation of the levels of glucose, lactate and uric acid in serum24 have been also recently reported. Alternative detection schemes, such as potentiometry, have shown some key advantages. Indeed, potentiometry has traditionally shown an unrivalled combination of robustness, simplicity of operation and low-cost of instrumentation. During the last few years, our group has pioneered the development of paper-based potentiometric sensors for the determination of ions in water and blood.25–27 Willander et al. expanded the development of low-cost potentiometric sensors for the determination of biomolecules such as glucose, cholesterol or uric acid.28,29 Despite the promising results of these devices, their validation against real samples has been scarce.
In a similar line, we have recently developed a novel approach to produce enzyme-based potentiometric sensors. Using a platinized paper as a substrate and a Nafion membrane to entrap the enzyme glucose-oxidase (GOx), a highly sensitive and selective sensor for glucose was developed (Fig. S1, ESI†).30 The system is based on the oxidation of glucose (eqn (1)) with the oxygen dissolved in the solution according to the following reaction:
| H2O + O2 + Glucose → Gluconolactone + H2O2 | (1) |
The platinum surface monitors the changes in the redox potential as a result of the generation of hydrogen peroxide:
| H2O2 → 2H+ + O2 + 2e− | (2) |
In a previous work30 we have demonstrated that the Nafion membrane has 3 different roles: first, it entraps the enzyme; second, it enhances the sensitivity to peroxide; third, it acts as a permselective barrier to minimize the effect of common interferences. The use of these sensors for the determination of glucose in biological fluids has been recently demonstrated.31 Furthermore, although illustrated for the case of glucose, this system is attractive because it can work as a generic detection platform for oxidase-type enzyme catalysed reactions. Nevertheless, to prove the real usefulness of this paper-based enzymatic platform, systems that can provide solutions to the whole analytical challenge – from sample introduction to data generation – are required.
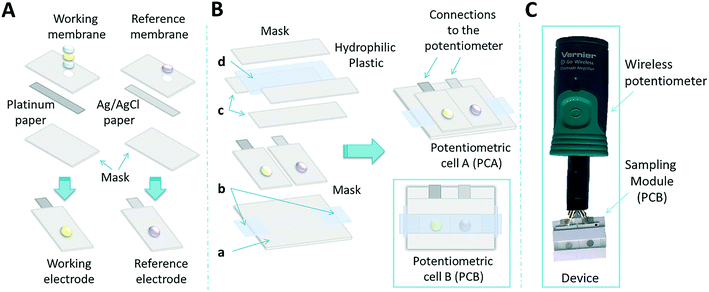
This work introduces for the first time a fully integrated, compact, portable and disposable wireless paper-based potentiometric system for detection of glucose in biological fluids such as serum and whole blood. Optimization of the sensitivity and selectivity of the working electrode is achieved by tuning the thickness of the Nafion layer and the cell design. After the development of the paper-based solid-state reference electrode, a complete potentiometric cell is presented (potentiometric cell A, PCA, Fig. 1). Thereafter, a suitable sampling module is designed to adapt to the potentiometric cell in order to reduce the sample volume to a single 25 μL drop (potentiometric cell B, PCB, Fig. 1). The system is first validated in the lab and then, through integration with a portable miniaturised wireless potentiometer, tested in real settings. The results show that this device displays a performance that is comparable to commercial glucometers, with the additional advantages of low cost, simplified detection scheme and low power consumption. Extensions of this platform to the determination of other targets are briefly introduced.
Experimental
Reagents
Whatman® Grade 5 qualitative filter paper was used for the fabrication of the electrodes. Nafion® perfluorinated resin solution (5 wt% in a mixture of lower aliphatic alcohols and water, 45% water), glucose oxidase (GOx) from Aspergillus niger type X-S, lyophilized powder, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–250
000–250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units per g solid, D-glucose, uric acid sodium salt, urea and sodium ascorbate were purchased from Sigma-Aldrich. Analytical-grade sodium hydrogen carbonate, potassium hydrogen phosphate, and chloride salts of potassium, sodium, magnesium and calcium were purchased from Sigma-Aldrich. All solutions were prepared using 18.2 MΩ cm−1 double deionized water (Milli-Q water systems, Merck Millipore). Butvar B-98 (PVB) was obtained from Quimidroga S.A. (Barcelona, Spain). Plastic mask (Arcare 8565) and highly hydrophilic plastic (ARflow® 92804) were provided by Adhesives Research Inc., Limerick, Ireland. Silver/silver chloride (Ag/AgCl) ink was purchased from Creative Materials Inc. (Massachusetts, USA).
000 units per g solid, D-glucose, uric acid sodium salt, urea and sodium ascorbate were purchased from Sigma-Aldrich. Analytical-grade sodium hydrogen carbonate, potassium hydrogen phosphate, and chloride salts of potassium, sodium, magnesium and calcium were purchased from Sigma-Aldrich. All solutions were prepared using 18.2 MΩ cm−1 double deionized water (Milli-Q water systems, Merck Millipore). Butvar B-98 (PVB) was obtained from Quimidroga S.A. (Barcelona, Spain). Plastic mask (Arcare 8565) and highly hydrophilic plastic (ARflow® 92804) were provided by Adhesives Research Inc., Limerick, Ireland. Silver/silver chloride (Ag/AgCl) ink was purchased from Creative Materials Inc. (Massachusetts, USA).
Phosphate buffered saline (PBS) was prepared at 0.1 M and used in all the experiments. Artificial serum samples contained 111 mM NaCl, 29 mM NaHCO3, 2.2 mM K2HPO4, 0.8 mM MgCl2 and 2.5 mM urea.32
Instrumentation and measurements
Lab measurements of the electromotive force (EMF) were performed with a high input impedance (1015 Ω) EMF16 multichannel data acquisition device (Lawson Laboratories, Inc. Malvern). A double junction Ag/AgCl/KCl 3 M reference electrode (type 6.0726.100, Metrohm AG) containing a 1 M LiAcO electrode bridge was used to optimise and compare the newly developed paper reference electrode. Laboratory measurements were made using a 4 mL cell in 0.1 M PBS (pH 7.4) and artificial serum (pH 7.4) at 25 °C. Wireless measurements of the sampling cell were obtained by Vernier Go Wireless® Electrode Amplifier with the Go Wireless app in a smartphone. N = 3, three different sensors in each experiment.A commercial amperometric glucose sensor (Bayer® Contour glucometer) was used to compare the glucose blood values determined by our paper-based potentiometric sensor and also to check the values from the local hospital obtained by colorimetric methods (hexokinase/glucose-6-phosphate dehydrogenase assay).
Platinum sputtering was performed using a radiofrequency sputtering process (ATC Orion 8-HV, AJA International) operated at 3 mTorr, for 65 s at 200 W in one side of a conventional filter paper.
Analysis of real samples
Serum samples of patients were obtained in a local hospital (Hospital Sant Joan de Deu, Barcelona). Values from serum samples were provided by the hospital using a hexokinase/glucose-6-phosphate dehydrogenase colorimetric test as a standard method for validation of the paper-based potentiometric system. Blood samples were obtained by the finger stick test from different volunteers. For the measurement of glucose levels in serum and blood samples, a dilution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in PBS (0.1 M, pH 7.4) was required. N = 3, three different sensors monitored each sample in this case.
10 in PBS (0.1 M, pH 7.4) was required. N = 3, three different sensors monitored each sample in this case.
All experiments were performed in compliance with the relevant laws and institutional guidelines. The work was conducted following strict protocols to guarantee the privacy and confidentiality of the data obtained. The work involved a continuous interaction with our partner medical center (Hospital Sant Joan de Deu, Barcelona) to guarantee safety and confidentiality, following the biosafety and security norms on this subject of the Universitat Rovira i Virgili. For this very small scale routine test, no specific approval of the committee was required. All the serum samples were obtained with informed consent of the patients.
Fabrication of the enzymatic paper-based potentiometric cell
As a first step, the preparation of the paper used as a substrate was performed. For the working electrodes, a 100 nm layer of platinum (Pt) was sputtered on one side of the filter paper to create a conductive, redox-sensitive surface. For the reference electrode, a filter paper was first painted with a conductive Ag/AgCl ink and cured for 10 minutes at 90 °C. These treated papers were then cut into 10 × 5 mm strips.To build the electrodes, the conductive paper strips were sandwiched within two plastic masks. The top mask has a circular window of 3 mm diameter to expose the electroactive surface, where the corresponding membrane (either for the working or for the reference) was drop cast (Fig. 1A). Further details of the electrode preparation have been described elsewhere.31
The working electrode was built in three steps: first, a volume of the Nafion solution was drop cast and dried at room temperature for one hour; second, 10 μL of a 20 mg mL−1 solution of glucose oxidase (GOx) were cast on top of the Nafion membrane and left to dry overnight at 4 °C; third, a volume of the Nafion solution was cast on top of the enzyme and left to dry overnight at 4 °C. Volumes of 2, 4.5, 7 and 9 μL of the Nafion solution were selected for the optimization. Two different cells with diameters of 3 mm (larger) and 2 mm (smallest) were evaluated. In each case, for the two additions, the volume was kept the same, i.e. 2 μL for the first and the second layer of Nafion for instance (see Table S3, ESI†). The experiments were performed with three sensors (SD, N = 3).
For the reference electrode,26 a total of 9 μL of a reference membrane consisting of 78 mg PVB and 50 mg NaCl in 1 mL of methanol was drop-cast (3 aliquots of 3 μL each, with 10 min drying at room temperature in between) and left to dry overnight. Thereafter, a first (and only) conditioning step of 8 h in 3 M KCl left the reference electrode ready for use.
To build the miniaturised cell (Fig. 1B), the enzymatic sensor was placed in between the sampling cell and connected to the measuring device through the conductive ends. This cell was kept at 4 °C when not in use.
The sampling cell was designed and optimised for a sample volume of 25 μL. This sampling module is made first with a plastic mask to accommodate the working and reference electrodes (Fig. 1Ba). Two strips made of a hydrophilic plastic material were placed at the end of the sensor to facilitate sample flow (Fig. 1Bb). Then, two plastic masks were placed around the electrodes to develop the sampling channel (Fig. 1Bc). Finally, a strip of hydrophilic plastic was placed on top of the sampling cell closing the microfluidic system (Fig. 1Bd). The last hydrophilic plastic was stacked with the plastic mask. The potentiometric cell integrated into the sampling cell had a size of 20 × 25 mm. Solution samples were placed in one entry and left to flow inside the channel driven by capillary forces. It is worth mentioning that the hydrophilic plastic improved the transport of the fluid through the sampling cell as well as the easy washing of the cell.
Results and discussion
Optimization of the potentiometric sensor
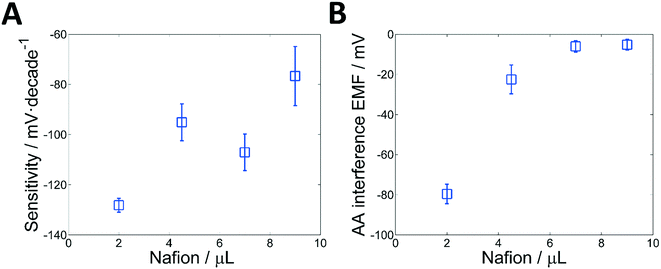
The detection of glucose is based on monitoring the change of the redox potential produced by the enzymatic reaction. Traditionally, Pt electrodes have been used to monitor the change in the redox potential of a solution, which can be produced by any redox-active substance (hydrogen peroxide among them). Thus, if a bare Pt electrode is used, the detection of the peroxide (H2O2) generated by the enzymatic reaction would be interfered by any other redox substance in the sample. In a previous work, however, we have demonstrated that when Pt electrodes are coated with a layer of Nafion, the selectivity towards peroxide is significantly enhanced, while a considerable increment in the sensitivity is observed.30 A typical time-trace illustrating this effect can be seen in Fig. 3A. The platinised paper acts as a redox-sensitive surface, while the layer Nafion has a dual role: it enhances the sensitivity of the detection and it increases the selectivity by restricting the effect of redox-active anions.31 This enhanced selectivity is due to the negatively charged nature of Nafion, which acts as a permselective barrier against interference of anions such as ascorbate and urate, i.e., typical redox-active species found in biological media. It has been shown that the improvement in sensitivity is linked to the Donnan potential, which is generated because of the ion-exchange capacity of Nafion.33,34 This phenomenon, however, is rather complex and several works have been devoted to characterize and understand the coupling between the redox and Donnan potential of these systems.35,36 In any case, this approach provides a very simple, sensitive and selective way to detect peroxide. In this case, a single enzyme and no additional reagents are required. Clearly, all the advantages mentioned are due to the coating of Nafion. Therefore, in order to gain further insights and optimize the response of the system, the influence of the thickness and casting of the Nafion layer on both the sensitivity and selectivity of the sensor was assessed.The construction of the electrode is based on the entrapment of the GOx, which is performed by drop-casting the Nafion (Fig. S1†) in two consecutive steps. Thus, experiments were performed where an increasing volume of Nafion – from 2 to 9 μL – was drop cast on the electrodes and the resulting sensitivity and interference from ascorbate were monitored (see Table S1, ESI† and the experimental part for details). In all cases, a linear range from 0.3–3 mM glucose (Fig. 2A) was obtained. The selectivity was measured by recording the change of electrode potential produced by the addition of ascorbic acid at 0.1 mM, which is usually considered the upper limit of the physiological levels in blood. The results are shown in Fig. 2B. This approach is analogous to one of the most used methods to determine the selectivity coefficient of ion-selective electrodes, i.e. the fix interference method (FIM).37,38
Interestingly, the results displayed in Fig. 2A show that increasing the amount of Nafion reduces the enhancement of the sensitivity. For example, the sensitivity (in mV per decade) was reduced from −128.2 down to −76.7 when the volume of Nafion cast was increased from 2 to 9 μL respectively. Since the electroactive area is constant, these results seem to suggest that the thinner the layer of Nafion, the greater the increment in sensitivity. However, a clear explanation of these observations is still under study, since many interdependent factors have to be considered. First, the way in which the Nafion dries may have an effect on the structure of the membrane. Second, the response of this system results from a complex coupling between the redox potential of H2O2 at the platinum electrode and the Donnan potential of the Nafion membrane. Thus, factors such as the local changes in pH, surface charges, etc., must be considered.
The effect of the amount of Nafion on the selectivity is shown in Fig. 2B. Unlike the sensitivity, the selectivity tends to increase as the amount of Nafion is increased. Although the properties and structure of Nafion are still a matter of study, it is well known that Nafion creates structures with negatively charged sulfonate groups in the sidechain forming nanopores, so that anionic molecules are unlikely to reach the electrode surface.39,40 This permselective behaviour is further enhanced as the thickness of the Nafion layer is increased.41–43 A maximum seems to be reached for a volume of membrane cast of approximately 7 μL, a point where a change of less than 5 mV is observed when the 0.1 mM ascorbate solution is added.
It is worth mentioning that the reproducibility between sensors is also affected by the amount of Nafion. In general, the higher the volume of Nafion, the larger the variation between sensors. This is probably due to the higher level of irreproducibility of the manual work.
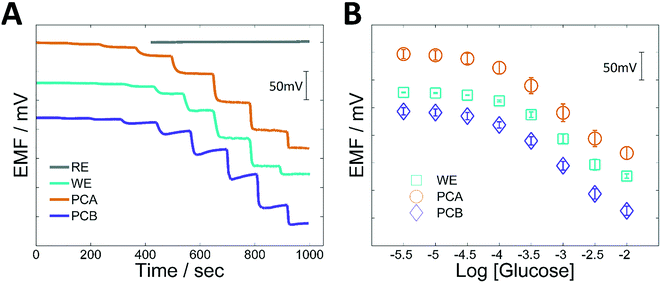
Because of the compromise between sensitivity and selectivity, the optimal amount of Nafion cast was set in 7 μL. Under these conditions, the working electrode exhibited a good sensitivity (−107.1 ± 7.2 mV log[glucose]1, N = 3) in the linear range (0.3–3 mM) and reduced level of interference. The most relevant analytical parameters are summarised in Table 1.
| Parameters | WE (PBS) | WE (artificial serum) | Potentiometric cell PCA (artificial serum) | Potentiometric cell PCB (artificial serum) |
|---|---|---|---|---|
| Sensitivity (mV per decade) | −107.1 ± 7.2 | −90.4 ± 4.9 | −95.8 ± 2.9 | −95.9 ± 4.8 |
| Linear range (mM) | 0.3–3 | 0.3–3 | 0.3–3 | 0.3–3 |
| LOD (mM) | 0.2 | 0.1 | 0.1 | 0.1 |
| Response time (s) | 50 | 60 | 60 | 80 |
It is well known that one of the key advantages of potentiometry is the possibility of miniaturization without affecting the analytical performance of the system. For this reason, experiments where the sensing area of the electrode was reduced in size were conducted. The results (Fig. S3, ESI†) are encouraging, since they show that the analytical parameters remain constant. However, a reduced window size (from 3 mm diameter to 2 mm diameter) increases the variability between electrodes, something that is most likely due to the error introduced by the manual deposition of the membranes.
All in all, the results show that these electrodes provide a sensitive and selective method for the direct potentiometric determination of glucose, with an unrivalled simplicity of construction and operation. While the sensitivity is fairly reproducible, the electrodes show a shifted potential response, a limitation that requires a calibration step. This is likely to be due to the manual method used to cast the membrane, a problem that can be minimized by using automated methods. A quick calculation of the cost of fabrication of the electrodes in the lab yields roughly 0.81 € per working electrode, 0.09 € per reference electrode and 1.05 € per sampling cell. This is, of course, a rough calculation that should be considered only as an upper limit to the cost, due to the off-the-shelf components and manual work. In terms of materials, the highest cost (so far) is due to the 100 nm Pt layer (a few micrograms) and the enzyme. Further optimization of the redox-sensitive surface will certainly improve this factor. Therefore, what is more important than the actual costs is that the techniques employed are scalable at the mass manufacturing level, which means that the cost could be reduced by several orders of magnitude.
A brief optimization of the reference electrode (RE) was also carried out. The most important step here is the conditioning step due to the presence of salts in the polymeric membrane.26 Fig. S4A (ESI†) displays the time-trace curve of three different electrodes in different conditioning times in 3 M KCl: 0 h, 3 h and at least 24 hours. The analytical performance of this paper RE was assessed by adding increasing concentrations of salts and monitoring the potential against a commercial RE. Hence, a calibration curve from 0.1 mM to 100 mM in double-distilled water was obtained. Results are presented in Fig. S4B, ESI.† An ideal behavior was obtained after a one time conditioning in 3 M KCl solution for 24 hours. Under these experimental conditions, the RE exhibited less than 0.14 mV per decade and a long term stability of 0.014 mV h−1 was obtained for a period of about 14 hours (Fig. S4C, ESI†). This paper-based reference electrode showed an outstanding performance with almost no potentiometric response and high stability and was used in the following section. Notably, the conditioning was only mandatory for the first time after fabrication, i.e. the electrodes could be dry stored and be ready for use afterwards.
Finally, in order to assess the influence of the matrix, calibrations performed in artificial serum were compared with those made in PBS. A slight reduction in sensitivity (from −107.1 ± 7.2 to −90.4 ± 4.9 mV per decade) but similar linear ranges and LODs were found (Table 1, Fig. 3). The time of response was also increased to approximately 60 s, but this is acceptable for real scenarios. This difference in sensitivity could be related to the change in the background solution (from PBS to artificial serum), since it has been already shown that the response of these systems is influenced by the total concentration of ions. Fortunately, biological fluids such as serum or blood have a relatively constant ion concentration, which allows one to perform the determination of glucose with major problems.
Analytical performance of the integrated devices
After their independent optimization, both working and reference electrodes were combined to build two potentiometric cells (PCA and PCB). In the first case (PCA), the electrodes were simply assembled together. In the second case (PCB), the electrodes were fitted with the “sampling module”, an arrangement where a hydrophilic plastic mask is placed over the electrodes in order to create a channel that reduces the required measuring volume down to 25 μL (Fig. 1B). Fig. 3 shows both time-traces and calibration curves for glucose in artificial serum for all these systems. In Fig. 3A the individual time traces for the WE and RE (separately), then for the PCA (in a 4 mL cell) and finally for the PCB (using only 25 μL of solution) are shown. Fig. 3B shows the corresponding calibration plots from 0.003–10 mM glucose. The results are shown in Table 1. Hence, similar parameters were obtained after integration of the WE and RE compared to the commercial reference electrode.These results show that the integration of the electrodes does not affect the performance of the sensors. Indeed, all the arrangements show a similar analytical performance. The most remarkable difference between the systems is the initial potential that makes the curves shifted by a constant value. Changes in the value of the reference electrode, as well as changes in the electrical characteristics of the cell will lead to this offset of the response, which is certainly not a problem if calibration is to be performed. Also, a difference in the time-trace is observed for PCB, i.e., when the electrodes are integrated into the miniaturized potentiometric cell. Likely, the incorporation of the sampling system might be altering the mass transport pattern and some electrical characteristics of the whole cell. For this reason, the response of PCB seems to be first dropping, and then taking slightly longer to stabilize. This time, however, it is not highly relevant from a practical point of view. Consequently, the system was validated using real samples.
Prediction of glucose in real samples
The validation of PCB was carried out using 10 (real) serum samples provided by a local hospital from different patients (diabetic and non-diabetic). To begin, the device was calibrated with two standards of glucose of 0.3–3 mM in PBS with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dilution of artificial serum, in order to simulate the conditions of real sample analysis. These two standards were chosen as they are the limits of the linear range of the sensor. Therefore, to monitor sugar in the physiological range of interest, a ten-fold dilution of the sample was required. After calibration, the cell was rinsed and then the dilution of the sample was added with a pipette until the whole sampling channel was filled (approximately 25 μL). After approximately 100 s, a steady-state signal was obtained. Thereafter, the sampling cell was rinsed thoroughly to remove any trace of the sample. The cleaning of the cell can be evaluated by checking that the open circuit potential returns to the initial value. This indicates that the system is ready to receive a new sample. This procedure, which was repeated using three different paper-based devices (Fig. 4), allows measuring 5 samples in about 6 minutes, including the calibration curve, washing, etc. For each sample, the protocol would be reduced to a couple of minutes.
10 dilution of artificial serum, in order to simulate the conditions of real sample analysis. These two standards were chosen as they are the limits of the linear range of the sensor. Therefore, to monitor sugar in the physiological range of interest, a ten-fold dilution of the sample was required. After calibration, the cell was rinsed and then the dilution of the sample was added with a pipette until the whole sampling channel was filled (approximately 25 μL). After approximately 100 s, a steady-state signal was obtained. Thereafter, the sampling cell was rinsed thoroughly to remove any trace of the sample. The cleaning of the cell can be evaluated by checking that the open circuit potential returns to the initial value. This indicates that the system is ready to receive a new sample. This procedure, which was repeated using three different paper-based devices (Fig. 4), allows measuring 5 samples in about 6 minutes, including the calibration curve, washing, etc. For each sample, the protocol would be reduced to a couple of minutes.
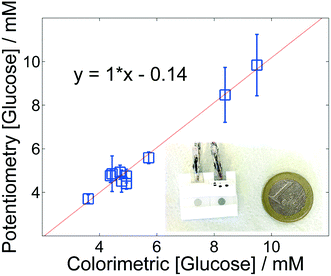
Fig. 4 shows the validation plot of the measurements using PCB vs. the values of glucose measured in the clinical lab of the hospital using the standard method hexokinase/glucose-6-phosphate dehydrogenase assay. These results show an encouraging correlation between the two methods (Table S2, ESI†). An average recovery of 100.7% (RSD 6.3, N = 10) was obtained, confirming the ability of PCB to accurately monitor glucose in serum. Nevertheless, at high concentrations the precision is reduced and we are currently exploring this point.
Instrumental advantages and future prospects
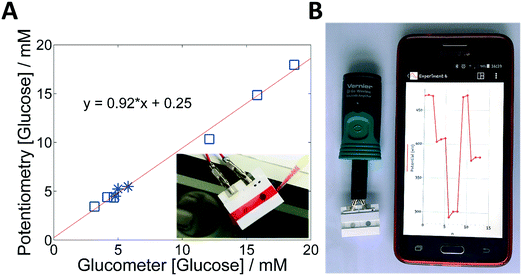
A simple and low cost sensor for glucose is only the first part. Instrumental aspects are also crucial when aiming to provide real solutions in decentralized settings with scarcity of resources. In this situation, potentiometry also offers significant advantages. From an electronic perspective, potentiometry requires extremely simple components, which makes the system cheap and less prone to noise and interferences. This is particularly relevant when dealing with systems that – following current trends in decentralized devices – have to be wirelessly connected to a central system where data can be uploaded. Furthermore, the overwhelming simplicity of operation makes the system easy to use with minimal training or expertise. To prove this point, a complete integration and miniaturization of the system was accomplished by using a high input impedance voltmeter with a Bluetooth data transmission system connected to a conventional smartphone. In this way, this kind of device should facilitate the remote management of chronic conditions, such as diabetes.Fig. 5 demonstrates the proof of concept of a PCB connected to a portable wireless data transmission device. The cell phone illustrates the time-trace plot created by the whole sensor system for one complete measurement including a two-point calibration, washing and sample measurement (Fig. 5B). Data was recorded wirelessly by Bluetooth® with the corresponding cell phone application and plotted real-time plot in the screen. The calibration curve yielded a linear relationship with a slope of −118 mV log[glucose]−1 over the 0.3–3 mM range.
The wireless system was also validated with whole blood and serum samples from diabetic patients, using the same protocol described above for PCB, i.e., using a ten-fold dilution of the samples in PBS. Fig. 5A shows the comparison between the wireless potentiometric system and a well-extended point-of-care (PoC) method, a commercially available glucometer. Potentiometry and amperometry techniques were indeed compared. Here, the glucometer did not require calibration nor dilution. An encouraging result was obtained using the potentiometric system and the standard PoC device. Moreover, Table S3 (ESI†) indicates the recovery of each sample giving an average of 97.3 (RSD 7.3, N = 9). Hence, validation using blood and serum samples demonstrated the proof of principle of the low-cost diagnostics device.
Conclusions
A simple, robust, low-cost and fully integrated potentiometric device for the determination of glucose in human serum and whole blood has been presented. The use of paper as a substrate for building the potentiometric cell combined with the Pt/Nafion interface provides a highly convenient way to detect the peroxide generated by the enzymatic reaction. Furthermore, the incorporation of a sample introduction channel facilitates the miniaturization of the system in order to reduce the volume of sample required. Finally, with the use of a simple electronic instrumentation, wireless collection of data is possible. This portable system was validated against standard lab-based methodologies as well as a standard point-of-care glucometer, confirming that this new platform could become a valuable tool in the growing fields of telemedicine and point-of-care.There are still many technological issues to be faced. From one side, current work is being focused on the control of the sensitivity and linear ranges of the system, in order to avoid dilutions or any other manipulation of the sample. Additionally, the work on the reproducibility of sensor manufacturing should allow the minimization (or even elimination) of the calibration steps.44,45 Finally, the extension of this approach to other oxidase-type enzymes open a new and exciting avenue for the development of low-cost, simple and robust platforms for decentralized analysis.46,47
Acknowledgements
The authors would like to acknowledge the financial support from Ramón y Cajal Programme, the Spanish Ministry of Economy and Competitiveness, European Regional Development Fund (ERDF) (Project CTQ2013-46404-R and CTQ2016-77128-R), FI (2016 FI_B1 00136) fellowship from Generalitat de Catalunya and Martí i Franqués Programme from Universitat Rovira i Virgili (2013PMF-PIPF-16). The authors would also like to thank Hospital de Sant Joan de Deu (Barcelona) for providing real samples.References
- E. Nolte, C. Knai and M. McKee, Obs. Stud. Ser. N˙15, 2008, pp. 1–202, ISBN 978 92 890 4294 9 Search PubMed.
- S. Nayak, N. R. Blumenfeld, T. Laksanasopin and S. K. Sia, Anal. Chem., 2016, 89, 102–123 CrossRef PubMed.
- A. J. Bandodkar, I. Jeerapan and J. Wang, ACS Sens., 2016, 1, 464–482 CrossRef CAS.
- J. L. Monteagudo, C. H. Salvador and L. Kun, Health Technol., 2014, 4, 79–93 CrossRef PubMed.
- N. P. Pai, C. Vadnais, C. Denkinger, N. Engel and M. Pai, PLOS Med., 2012, 9, 1–7 Search PubMed.
- A. Nemiroski, D. C. Christodouleas, J. W. Hennek, A. A. Kumar, E. J. Maxwell, M. T. Fernández-Abedul and G. M. Whitesides, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 1–6 CrossRef PubMed.
- D. E. Goldstein, R. R. Little, R. A. Lorenz, J. I. Malone, D. Nathan, C. M. Peterson and D. B. Sacks, Diabetes Care, 2004, 27, 1761–1773 CrossRef PubMed.
- J. C. N. Chan, R. Y. M. Wong, C. K. Cheung, P. Lam, C. C. Chow, V. T. F. Yeung, E. C. Y. Kan, K. M. Loo, M. Y. L. Mong and C. S. Cockram, Diabetes Res. Clin. Pract., 1997, 36, 91–104 CrossRef CAS PubMed.
- B. Solnica, J. W. Naskalski and J. Sieradzki, Clin. Chim. Acta, 2003, 331, 29–35 CrossRef CAS.
- E. J. Maxwell, A. D. Mazzeo and G. M. Whitesides, MRS Bull., 2013, 38, 309–314 CrossRef CAS.
- J. E. Shaw, R. A. Sicree and P. Z. Zimmet, Diabetes Res. Clin. Pract., 2010, 87, 4–14 CrossRef CAS PubMed.
- H. King, R. E. Aubert and W. H. Herman, Diabetes Care, 1998, 21, 1414–1431 CrossRef CAS PubMed.
- S. K. Vashist, Anal. Chim. Acta, 2012, 750, 16–27 CrossRef CAS PubMed.
- C. McCormick, D. Heath and P. Connolly, Sens. Actuators, B, 2012, 166–167, 593–600 CrossRef CAS.
- A. W. Martinez, S. T. Phillips, M. J. Butte and G. M. Whitesides, Angew. Chem., Int. Ed., 2007, 46, 1318–1320 CrossRef CAS PubMed.
- J. L. Delaney, C. F. Hogan, J. Tian and W. Shen, Anal. Chem., 2011, 83, 1300–1306 CrossRef CAS PubMed.
- S. Chen, Q. Wan and A. K. Badu-Tawiah, J. Am. Chem. Soc., 2016, 138, 6356–6359 CrossRef CAS PubMed.
- Z. Nie, C. A. Nijhuis, J. Gong, X. Chen, A. Kumachev, A. W. Martinez, M. Narovlyansky and G. M. Whitesides, Lab Chip, 2010, 10, 477–483 RSC.
- V. F. Curto, N. Lopez-Ruiz, L. F. Capitan-Vallvey, A. J. Palma, F. Benito-Lopez and D. Diamond, RSC Adv., 2013, 3, 18811 RSC.
- S. N. Tan, L. Ge, H. Y. Tan, W. K. Loke, J. Gao and W. Wang, Anal. Chem., 2012, 84, 10071–10076 CrossRef CAS PubMed.
- C. Desmet, C. A. Marquette, L. J. Blum and B. Doumèche, Biosens. Bioelectron., 2016, 76, 145–163 CrossRef CAS PubMed.
- D. M. Cate, J. A. Adkins, J. Mettakoonpitak and C. S. Henry, Anal. Chem., 2015, 87, 19–41 CrossRef CAS PubMed.
- J. Mettakoonpitak, K. Boehle, S. Nantaphol, P. Teengam, J. A. Adkins, M. Srisa-Art and C. S. Henry, Electroanalysis, 2016, 28, 1420–1436 CrossRef CAS.
- W. Dungchai, O. Chailapakul and C. S. Henry, Anal. Chem., 2009, 81, 5821–5826 CrossRef CAS PubMed.
- M. Novell, M. Parrilla, G. A. Crespo, F. X. Rius and F. J. Andrade, Anal. Chem., 2012, 84, 4695–4702 CrossRef CAS PubMed.
- T. Guinovart, G. A. Crespo, F. X. Rius and F. J. Andrade, Anal. Chim. Acta, 2014, 821, 72–80 CrossRef CAS PubMed.
- M. Novell, T. Guinovart, P. Blondeau, F. X. Rius and F. J. Andrade, Lab Chip, 2014, 14, 1308–1314 RSC.
- M. Willander, K. Khun and Z. H. Ibupoto, Sensors, 2014, 14, 8605–8632 CrossRef PubMed.
- M. H. Asif, B. Danielsson and M. Willander, Sensors, 2015, 15, 11787–11804 CrossRef CAS PubMed.
- M. Parrilla, R. Cánovas and F. J. Andrade, Electroanalysis, 2016, 29, 223–230 CrossRef.
- M. Parrilla, R. Cánovas and F. J. Andrade, Biosens. Bioelectron., 2017, 90, 110–116 CrossRef CAS PubMed.
- K. Kimura, H. Oishi, T. Miura and T. Shono, Anal. Chem., 1987, 59, 2331–2334 CrossRef CAS PubMed.
- R. Das and G. H. Pollack, Langmuir, 2013, 29, 2651–2658 CrossRef CAS PubMed.
- K. Kanamura, H. Morikawa and T. Umegaki, J. Electrochem. Soc., 2003, 150, A193–A198 CrossRef CAS.
- R. Naegeli, J. Redepenning and F. C. Anson, J. Phys. Chem., 1986, 90, 6227–6232 CrossRef CAS.
- E. J. Calvo and A. Wolosiuk, J. Am. Chem. Soc., 2002, 124, 8490–8497 CrossRef CAS PubMed.
- E. Bakker, E. Pretsch and P. Bühlmann, Anal. Chem., 2000, 72, 1127–1133 CrossRef CAS PubMed.
- E. Bakker, Electroanalysis, 1997, 9, 7–12 CrossRef CAS.
- K. A. Mauritz and R. B. Moore, Chem. Rev., 2004, 104, 4535–4585 CrossRef CAS PubMed.
- K. Schmidt-Rohr and Q. Chen, Nat. Mater., 2008, 7, 75–83 CrossRef CAS PubMed.
- B. D. Bath, H. S. White and E. R. Scott, Anal. Chem., 2000, 72, 433–442 CrossRef CAS PubMed.
- E. J. Calvo and A. Wolosiuk, J. Am. Chem. Soc., 2002, 124, 8490–8497 CrossRef CAS PubMed.
- M. A. Izquierdo-Gil, V. M. Barragán, J. P. G. Villaluenga and M. P. Godino, Chem. Eng. Sci., 2012, 72, 1–9 CrossRef CAS.
- X. U. Zou, X. V. Zhen, J. H. Cheong and P. Bühlmann, Anal. Chem., 2014, 86, 8687–8692 CrossRef CAS PubMed.
- U. Vanamo and J. Bobacka, Anal. Chem., 2014, 86, 10540–10545 CrossRef CAS PubMed.
- E. Witkowska Nery, M. Kundys, P. S. Jeleń and M. Jönsson-Niedziółka, Anal. Chem., 2016, 88, 11271–11282 CrossRef CAS PubMed.
- H. Lee, T. K. Choi, Y. B. Lee, H. R. Cho, R. Ghaffari, L. Wang, H. J. Choi, T. D. Chung, N. Lu, T. Hyeon, S. H. Choi and D. H. Kim, Nat. Nanotechnol., 2016, 11, 566–572 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7lc00339k |
| This journal is © The Royal Society of Chemistry 2017 |