Open Access Article
Open Access ArticleA derivatisation agent selection guide†
Marek
Tobiszewski
 a,
Jacek
Namieśnik
a and
Francisco
Pena-Pereira
a,
Jacek
Namieśnik
a and
Francisco
Pena-Pereira
 *b
*b
aDepartment of Analytical Chemistry, Chemical Faculty, Gdańsk University of Technology (GUT), 11/12 G. Narutowicza St., 80-233 Gdańsk, Poland
bDepartment of Analytical and Food Chemistry, Faculty of Chemistry, University of Vigo, Campus As Lagoas – Marcosende s/n, 36310 Vigo, Spain. E-mail: fjpena@uvigo.es
First published on 17th November 2017
Abstract
The study reported herein is aimed at the greenness assessment of 267 derivatisation agents that are frequently applied in analytical chemistry and related disciplines. Multicriteria decision analysis allowed obtaining three rankings of derivatisation agents applied in liquid chromatography, gas chromatography and chiral analysis. The criteria of assessment included the safety information obtained from material safety data sheets and physicochemical and environmental parameters predicted with relevant models. As for some of the agents predicted data were not available, these agents were assessed with a smaller number of criteria, within the ranking of low confidence. The results of the study will help to apply greener derivatisation agents, wherever the green chemistry principle of avoiding derivatisation cannot be fulfilled.
1. Introduction
The development of new tools for providing high quality information in a cost-effective and expeditious way is one of the main aims of analytical chemistry. Remarkably, the introduction of the 12 principles of green chemistry1 paved the way forward for the development of analytical methodologies that are, ideally, inherently safe for the operator and the environment, with the least possible consumption of energy and chemicals, and minimum generation of wastes.2,3 Thus, green aspects are increasingly being considered besides the main features of analytical methods such as accuracy, sensitivity, selectivity and precision.3 In this way, both “3R” (reduction, replacement and recycling)4 and “4S” (specific methods, smaller dimensions, simpler methods and statistics)5 approaches have been reported in the literature towards greener analytical methodologies.2The removal, replacement by greener alternatives or minimisation of reagents and solvents used in chemical processes is recommended in several of the 12 principles of green chemistry.1 While certain strategies have enabled reagentless and solventless processes, many scientific and technological activities require significant amounts of both solvents and chemicals. In the latter case, selection of solvents and chemicals with little (or none) environmental, health and safety (EHS) issues is highly recommended. In this sense, a number of solvent selection guides have been developed in recent years, enabling the selection of greener alternatives to harmful solvents typically used in scientific and technological processes.6–14 The possibility of developing reagent selection guides has been however much less explored, probably due to the additional EHS issues associated with these non-inert chemicals and lack of physicochemical data, among other aspects.8 Notwithstanding the above, both Pfizer and GSK have made remarkable efforts towards the selection of greener reagents among the ones used in common transformations by the pharmaceutical industry.15–17 Specifically, Alfonsi et al.8 first introduced the concept of a reagent guide almost one decade ago and, more recently, scientists at GSK developed a selection guide for reagents used in a wide range of transformations in the pharmaceutical industry,16 as well as a selection guide for greener acids and bases.17 Even though the above mentioned guides cover a broad range of chemicals used in medicinal chemistry and the pharmaceutical industry, further progress is necessary to provide valuable information to other fields where derivatisation reactions are required.
Multicriteria decision analysis (MCDA) consists of a set of tools for solving complex decision problems. A result of the analysis of a dataset of alternatives, described by criteria, is a selection of the first preference solution and creation of the full ranking of the remaining alternatives. MCDA has been applied in sustainability assessments18 and environmental managerial processes.19 More specifically, MCDA has been used to rank solvents according to their greenness12 and environmental risks related to their emissions.13 Other chemical assessment applications include screening amine-based solvents for CO2 capture20 or screening of more sustainable substitutes for chemicals, based on the quantitative structure–use relationship.21
The term derivatisation refers to a chemical reaction that aims to change the chemical structure of target compounds and, as a consequence, obtain derivatives with desirable physicochemical properties for separation and/or detection. Ideally, derivatisation should be quantitative and selective, and fast and simple to perform. The 8th principle of green chemistry states that unnecessary derivatisation should be minimised or avoided whenever possible in order to avoid or minimise reagent consumption and the corresponding waste generation.1 Thus, a widely employed strategy in synthetic organic chemistry such as the use of blocking or protecting groups (or any temporary modifications) should be avoided to reduce its impact on the process mass intensity. Even though the complete removal of derivatisation agents is the ideal solution, it should be borne in mind that their use in analytical chemistry has enabled the determination of a wide range of compounds not directly amenable to analysis, among other performance benefits. Particularly, analytical derivatisation can enhance detection and separation, extractability, thermal stability, selectivity, and the overall quality of the data.22–24 Thus, a wide range of disciplines, e.g., chemical, biochemical, medical, forensic and environmental sciences, make use of analytical derivatisation.22,23 Selection of greener derivatisation agents would therefore help in the development of less harmful methodologies where derivatisation reactions cannot be avoided.
The work reported herein focuses on the development of a reagent selection guide to rank derivatisation reagents relevant to analytical chemistry and related disciplines taking into account their EHS concerns. We believe that the greenness assessment of derivatisation agents performed in this work will help to focus future efforts in the replacement of the chemicals which show major issues by greener alternatives.
2. Materials and methods
2.1. Data collection
The dataset describing commercially available derivatisation agents (n = 267) was created (see the ESI†). Each derivatisation agent was described by 13 assessment criteria as presented in Table 1. The data were taken from material safety data sheets (MSDS) of the respective compounds and from modelling results available at ChemSpider webpage.25 Derivatisation agents were divided into three subsets, according to their area of application, namely liquid chromatography (LC) derivatisation agents (n = 133), gas chromatography (GC) derivatisation agents (n = 98) and chiral derivatisation agents (n = 36). This grouping was based on statements “for LC derivatisation”, “for GC derivatisation” or “for chiral derivatisation” from the Sigma-Aldrich web page.| Criterion | Description | Weight |
|---|---|---|
| Boiling point | From the ChemSpider web page. Experimental data were taken if available. If not, data predicted by ACD/Labs or EPISuite models were applied | 0.025 |
| Flash point | 0.025 | |
| Vapour pressure | 0.025 | |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW KOW |
0.025 | |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOC KOC |
0.025 | |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BCF BCF |
0.025 | |
| Total removal by wastewater treatment (%) | 0.025 | |
| Persistence time | 0.025 | |
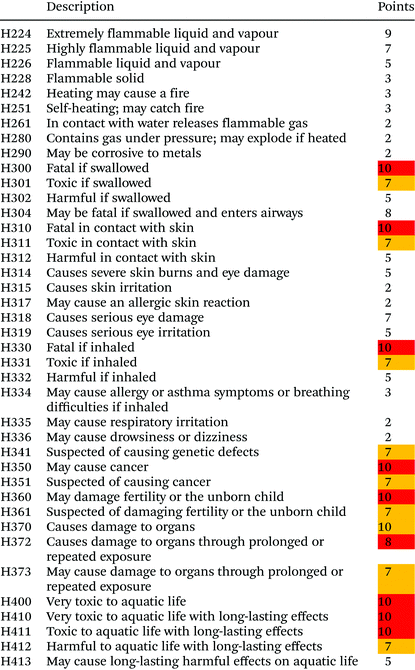
| Hazard statements (H) | From MSDS, SECTION 2: Hazards identification, 2.2 Label elements. For translation of statements to numerical values see Table 2 | 0.25 (0.3125) |
| Precautionary statements (P) | From MSDS, SECTION 2: Hazards identification, 2.2 Label elements. For translation of statements to numerical values see Table 3 | 0.2 (0.25) |
| Carcinogenicity (IARC) | From MSDS, SECTION 11: Toxicological information, information on toxicological effects. Group 2A: Probably carcinogenic to humans – 3 points; not identified as a probable, possible or confirmed human carcinogen – 0 points | 0.05 (0.625) |
| Signal word | From MSDS, SECTION 2: Hazards identification, 2.2 Label elements. “None” – 0 points, “Warning” – 1 point, “Danger” – 4 points | 0.2 (0.25) |
| Special hazards arising from the substance or mixture/hazardous decomposition products | From MSDS, SECTION 5: Firefighting measures, 5.2 Special hazards arising from the substance or mixture, SECTION 10: Stability and reactivity, 10.6 Hazardous decomposition products. For translation of statements to numerical values see Table 4 | 0.1 (0.125) |
The required data for some of the evaluated derivatisation agents were not available on the ChemSpider web page. Rankings of lower confidence were thus prepared in order to include them in the assessment procedure. High confidence rankings were prepared on the basis of all criteria presented in Table 1. Low confidence rankings were based on hazard statements, precautionary statements, carcinogenicity, signal wording and special hazards arising from the substance or mixture/hazardous decomposition products criteria.
2.2. Assessment criteria
The sources of the data of the respective criteria are listed in Table 2. The application of MCDA tools requires defining preference functions for every single criterion. They are usually defined as “the higher the better” or “the lower the better”. The first preference function was applied for the boiling point, flash point and total removal by wastewater treatment. The rest of the criteria were evaluated according to “the lower the better” preference function.MCDA methods offer the possibility to assign different relative importance to the respective criteria in the form of weights. In high confidence rankings, low weights were given to criteria referring to physicochemical parameters, persistence time and removal during wastewater treatment. These data are mostly predicted by models, so their reliability is lower than that of measured values. These criteria mostly characterise risks related to the environmental fate of chemicals, not the exposure or occupational risks. The data that are available in MSDS are given higher weights as these criteria directly reflect the risks connected with the application of derivatisation agents. Here, hazard statements, precautionary statements and signal wording characterise application risks. Carcinogenicity is given smaller weight as this criterion presents little variability, since only 2 derivatisation agents are considered possible human carcinogens. For low confidence ranking, the ratios between weights remained unchanged in comparison with high confidence ranking.
The data being input to TOPSIS (Technique for Order of Preference by Similarity to Ideal Solution) must be in the form of numerical values. Therefore, hazard statements and precautionary statements were transformed to penalty points in 10 point scales. Points for hazard statements are presented in Table 2. Ten points were given to hazard statements with zero pass and first pass red flags as presented in a recently proposed unified metrics toolkit for the assessment of the sustainability of reactions.26 First pass amber flags statements from this assessment were given seven points in our study. Red and amber flags statements are marked in Table 2 with appropriate colours. The rest of the statements got penalty points according to the risks related to their descriptions. Derivatisation agents with multiple hazard statements got their points summed up to obtain a final numerical value of this criterion.
Points for precautionary statements are presented in Table 3. The points for this criterion were given to the respective statements in a similar way to the case of hazard statements according to their risks. The points for derivatisation agents with multiple precautionary statements were summed up to obtain a numerical value of this criterion.
| Precautionary statement (P) | Description | Points |
|---|---|---|
| P201 | Obtain special instructions before use | 10 |
| P210 | Keep away from heat/sparks/open flames/hot surfaces – no smoking | 3 |
| P231 | Handle under inert gas | 7 |
| P232 | Protect from moisture | 3 |
| P233 | Keep the container tightly closed | 3 |
| P235 | Keep cool | 3 |
| P260 | Do not breathe dust/fume/gas/mist/vapours/spray | 7 |
| P261 | Avoid breathing dust/fume/gas/mist/vapours/spray | 5 |
| P264 | Wash hands thoroughly after handling | 3 |
| P273 | Avoid release to the environment | 10 |
| P280 | Wear protective gloves/protective clothing/eye protection/face protection | 5 |
| P284 | Wear respiratory protection | 10 |
| P301 | IF SWALLOWED: | 5 |
| P302 | IF ON SKIN: | 6 |
| P303 | IF ON SKIN (or hair): | 7 |
| P304 | IF INHALED: | 10 |
| P305 | IF IN EYES: | 7 |
| P308 | IF exposed or concerned: | 5 |
| P310 | Immediately call a POISON CENTER or doctor/physician | 10 |
| P311 | Call a POISON CENTER or doctor/physician | 7 |
| P312 | Call a POISON CENTER or doctor/physician if you feel unwell | 3 |
| P313 | Get medical advice/attention | 2 |
| P330 | Rinse mouth | 5 |
| P331 | Do NOT induce vomiting | 5 |
| P337 | If eye irritation persists: | 5 |
| P338 | Remove contact lenses, if present and easy to do. Continue rinsing | 5 |
| P340 | Remove the victim to fresh air and keep at rest in a position comfortable for breathing | 3 |
| P342 | If experiencing respiratory symptoms: | 7 |
| P350 | Gently wash with plenty of soap and water | 3 |
| P351 | Rinse cautiously with water for several minutes | 3 |
| P352 | Wash with plenty of soap and water | 3 |
| P353 | Rinse skin with water/shower | 3 |
| P361 | Remove/take off immediately all contaminated clothing | 3 |
| P370 | In case of fire: | 7 |
| P378 | Use … for extinction | 5 |
| P391 | Collect spillage | 5 |
| P403 | Store in a well-ventilated place | 3 |
| P405 | Store locked up | 2 |
| P410 | Protect from sunlight | 3 |
| P501 | Dispose of contents/container to … | 5 |
Signal wording transformation to obtain numerical values, “none” – 0 points, “warning” – 1 point, “danger” – 4 points, was also used in the transformation of “special hazards arising from the substance or mixture/hazardous decomposition products” into numerical values, as shown in Table 4. Following the approach of the analytical eco-scale,27 the points for signal wording were multiplied by the number of labelling pictograms. For compounds with (+) indication, extra 10 points were given as these compounds are characterised by hazards with lethal effects. For multiple compounds formed during fire or decomposition, their points were summed up to obtain the final value of the criterion.
| Compounds | No pictograms | Signal wording | Points |
|---|---|---|---|
| Carbon oxides | 1 | Warning | 1 |
| Nitrogen oxides | 4 | Danger | 16 |
| Sulphur oxides | 3 | Danger | 12 |
| Phosphorus oxides | 1 | Danger | 4 |
| Borane/boron oxides | 1 | Danger | 4 |
| Iron oxides | 0 | 0 | 0 |
| Sodium oxides | 2 | Danger | 8 |
| Silicon oxides | 0 | 0 | 0 |
| Hydrogen chloride | 2 | Danger | 8 |
| Hydrogen bromide | 3 | Danger | 12 |
| Hydrogen iodide | 1 | Danger | 4 |
| Hydrogen fluoride | 2 | Danger (+) | 18 |
| Hydrogen cyanides | 3 | Danger (+) | 22 |
| Diazomethane | 3 | Danger | 12 |
| Dimethylamine | 3 | Danger | 12 |
2.3. TOPSIS analysis
The algorithm of TOPSIS requires few simple steps to calculate the final ranking. The step one is to normalize the input data to form a “normalised decision matrix”. Normalised value rxy is calculated according to the equation: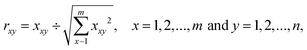 | (1) |
Then, the weighted normalised decision matrix is calculated. The weighted normalised values νxy are calculated according to the equation:
| νxy = rxy × wy x = 1,2,…,m and y = 1,2,…,n, | (2) |
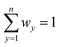 . In this study, the applied weights are presented in Table 1.
. In this study, the applied weights are presented in Table 1.
In the next step, positive ideal solution (A*) and negative ideal solution (A−) are calculated with the following equations:
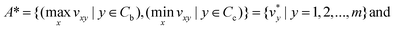 | (3) |
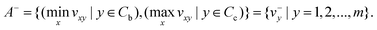 | (4) |
The separation measures using the m-dimensional Euclidean distance are determined in the next step. The separation measures of each alternative from the positive 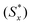 and negative
and negative 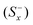 ideal solutions, respectively, are calculated as follows:
ideal solutions, respectively, are calculated as follows:
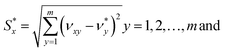 | (5) |
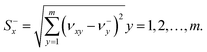 | (6) |
Then the relative distance to the ideal solution is calculated. The relative distance of the alternative Ax with respect to A* is defined as:
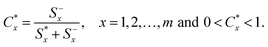 | (7) |
The alternative with 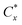 closest to 1 is called the best preference. All alternatives are characterised by the values of similarity to the ideal solution and the ranking is obtained. The rankings for all three groups of derivatisation agents are provided in Tables 5–7.
closest to 1 is called the best preference. All alternatives are characterised by the values of similarity to the ideal solution and the ranking is obtained. The rankings for all three groups of derivatisation agents are provided in Tables 5–7.
3. Results and discussion
As stated in the above section, derivatisation agents were assessed within three groups. The assessment results for LC, GC, and chiral derivatisation agents are presented in the next three subsections. It should be noted here that the results for derivatisation agents present in the different subsets are incomparable. As the assessment results for high confidence rankings are based on more assessment criteria, it is advisable to consider these results. If not available, low confidence ranking brings approximate information on the performance of derivatisation agents. Another important information is the assessment score, elegantly named “similarity to ideal solution”. The value equal to “1” means that all assessment criteria for this derivatisation agent are characterised by the best performance from the dataset. In contrast, a value equal to “0” means that all assessment criteria for a given derivatisation agent are characterised by the worst performance from the dataset. The values between “1” and “0” indicate how the alternative is similar or dissimilar to the ideal solution. For easier decision making, the signal word information of each derivatisation agent is included in Tables 5–7 and expressed with colours. “None” signal wording is highlighted in green, “warning” in amber and “danger” in red. In general terms, green coloured agents with “none” wording are ranked high and red “danger” agents are ranked low, as can be observed in Tables 5–7.3.1. LC derivatisation agent assessment
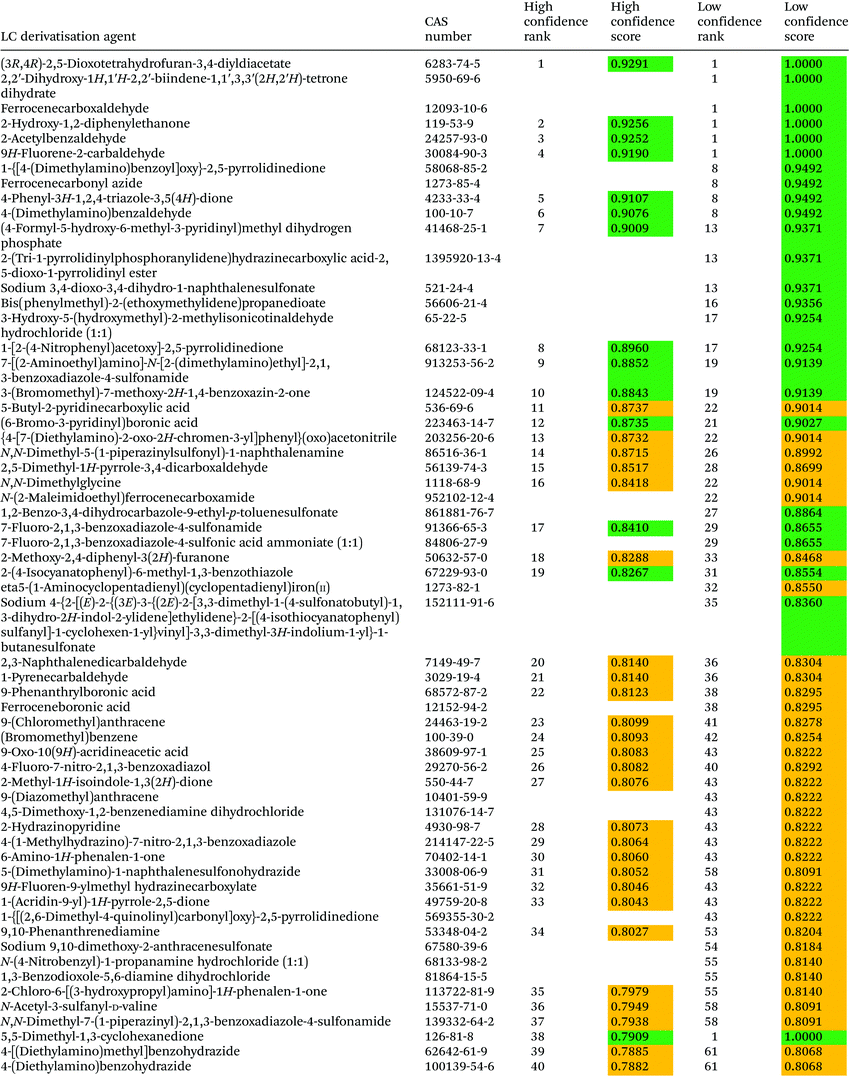
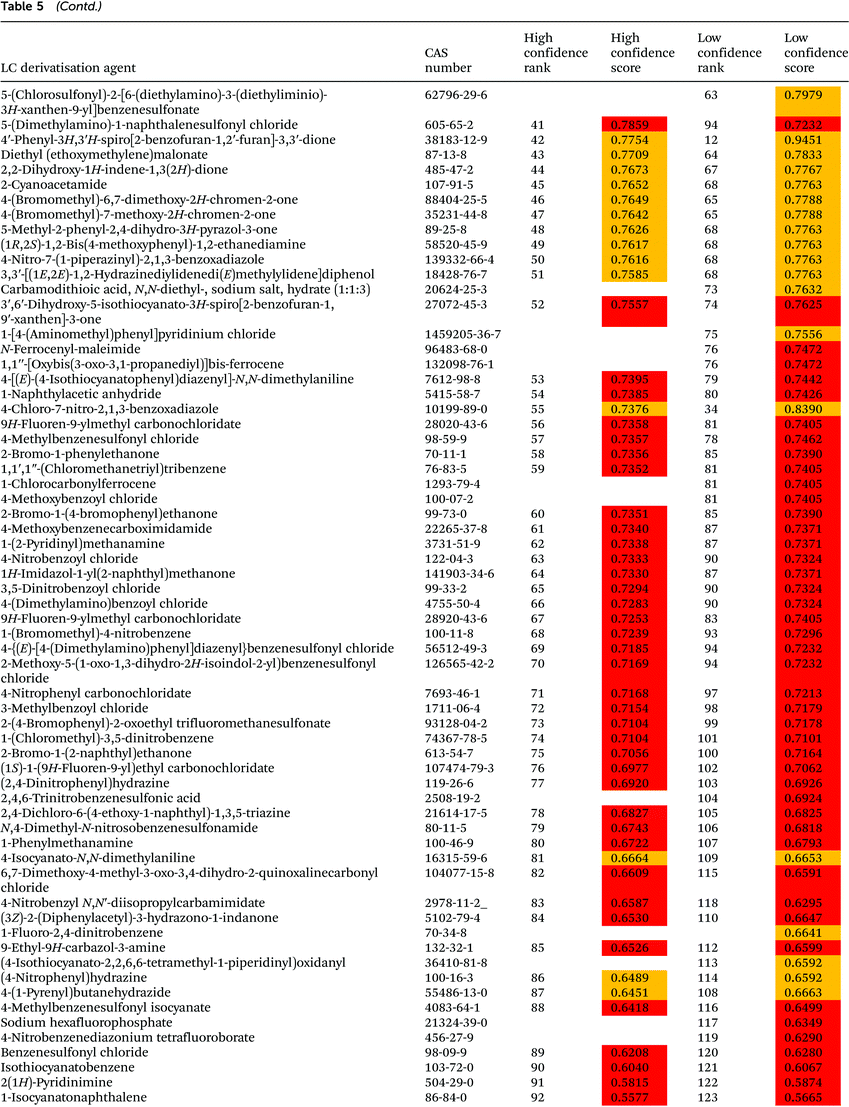
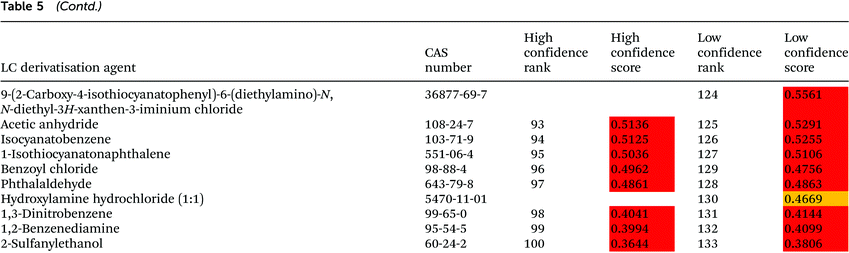
The ranking of 133 LC derivatisation agents is presented in Table 5. According to the low confidence ranking, seven derivatisation agents, namely (3R,4R)-2,5-dioxotetrahydrofuran-3,4-diyldiacetate, 2,2′-dihydroxy-1H,1′H-2,2′-biindene-1,1′,3,3′(2H,2′H)-tetrone dihydrate, ferrocenecarboxaldehyde, 2-hydroxy-1,2-diphenylethanone, 2-acetylbenzaldehyde, and 9H-fluorene-2-carbaldehyde, are equally ranked as the 1st preference. The high confidence ranking includes only five out of these seven agents, and some differences between these agents are noticeable. Thus, 5,5-dimethyl-1,3-cyclohexanedione – the first rank according to low confidence ranking – is still within the first tertile but ranked 38th with high confidence ranking due to its relatively low boiling point and flash point, reduced removal during wastewater treatment and longer environmental residence time. 2-Hydroxy-1,2-diphenylethanone, a derivatisation agent also used as a food additive for human consumption,28 remarkably ranked 2nd with high confidence ranking. 4-(Dimethylamino)benzaldehyde, a derivatisation agent of amino acids and peptides22,29 known as Ehrlich’ reagent, ranked 6th with high confidence ranking.Remarkably, 8 out of 9 LC derivatisation agents included as restricted and priority substances in SUBSPORT,30 SIN List,31 and/or the EPA's Toxics Release Inventory (TRI) chemicals list32 are ranked at the very bottom of the high confidence ranking (85–100). These chemicals included 9-ethyl-9H-carbazol-3-amine, benzenesulfonyl chloride, acetic anhydride, isocyanatobenzene, benzoyl chloride, hydroxylamine hydrochloride, 1,3-dinitrobenzene, and 1,2-benzenediamine, all of them labelled as dangerous compounds. (Bromomethyl)benzene is, on the other hand, an acylating agent identified with the signal word “warning” that has been ranked 24th by high confidence ranking. Even though this substance is not included in the SIN List and TRI chemicals list, it is not allowed in BSH products.30
The last agent in the ranking, 2-sulfanylethanol, is characterised by serious hazard and precautionary statements, including fatal effects. Characteristics of neighbouring agents are similar to 2-sulfanylethanol. Benzoyl chloride (rank 96 by the high confidence procedure) is even indicated by IARC as a probable human carcinogen. Benzoyl chloride is used for the derivatisation of hydroxyl-bearing compounds.33,34 Phthalaldehyde (rank 97, according to high confidence ranking) is a derivatisation agent used for amine and thiol derivatisation34,35 characterized by high toxicity towards rats (oral LD50 value equal to 178 mg kg−1). Isocyanatobenzene (rank 94 with high confidence ranking), used for derivatisation of amines and hydroxyl-bearing compounds,36–38 is not highly toxic towards rats (oral LD50 = 800 mg kg−1), but is highly toxic towards rats through inhalation in a 4 h test (LC50 = 22 mg m−3) and toxic towards Danio rerio in a 96 h test (LC50 = 84 mg L−1).
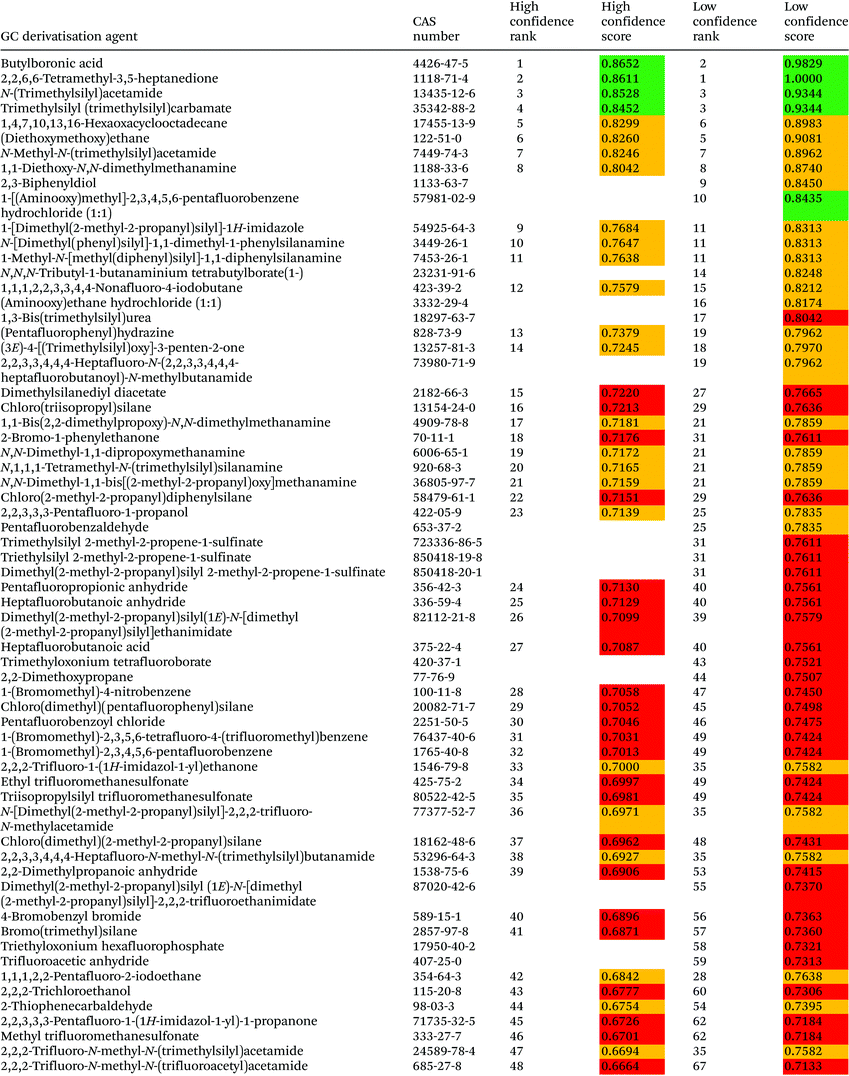
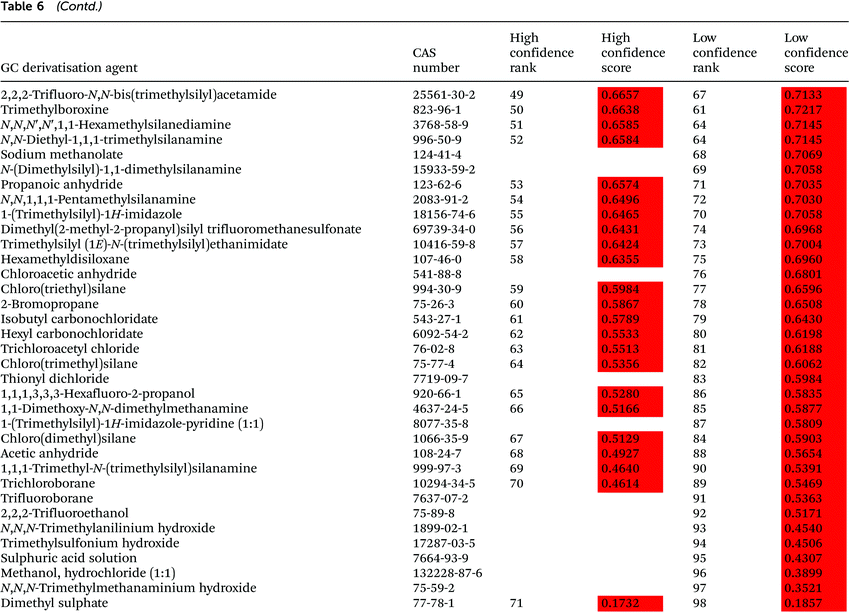
3.2. GC derivatisation agent assessment
The assessment of GC derivatisation agents was performed with 98 compounds, and the obtained ranking is provided in Table 6. Most of the GC derivatisation agents considered in the study are used in alkylation/esterification (43%) and silylation (39%) reactions, whereas the remaining 18% are used as acylation agents. Five silylation agents, namely N-(trimethylsilyl)acetamide, trimethylsilyl (trimethylsilyl)carbamate, N-methyl-N-(trimethylsilyl)acetamide, 1-[dimethyl(2-methyl-2-propanyl)silyl]-1H-imidazole and N-[dimethyl(phenyl)silyl]-1,1-dimethyl-1-phenylsilanamine, four alkylation/esterification agents, namely butylboronic acid, 1,4,7,10,13,16-hexaoxacyclooctadecane, (diethoxymethoxy)ethane and 1,1-diethoxy-N,N-dimethylmethanamine, and one acylation agent, namely 2,2,6,6-tetramethyl-3,5-heptanedione, where ranked among the first 10 GC derivatisation agents in accordance with the high confidence ranking. The highest rank of fluorinated compounds (1-[(aminooxy)methyl]-2,3,4,5,6-pentafluorobenzene hydrochloride) is 10 according to the low confidence ranking.The least preferable derivatisation agent according to both rankings is dimethyl sulphate, a chemical used in alkylation and esterification reactions that is characterised by many hazard and precautionary statements and categorised by IARC as a probable human carcinogen. This compound is characterised by high toxicity in rats with an LC50 = 45 mg m−3 in a 4 hour-exposure test and towards Lepomis macrochirus with an LC50 = 7.5 mg L−1 in a 96 h test, as stated in MSDS. Also commonly applied derivatisation agents, such as a methanol and hydrochloric acid mixture or sulphuric acid are ranked very low, although they are assessed with the low confidence ranking only.
Acetic anhydride (rank 68 according to the high confidence ranking) is a commonly applied acylating agent in GC determinations. It is well characterised in terms of its toxicological parameters, oral toxicity towards rats (LD50 = 630 mg kg−1), inhalation toxicity towards rats in a 6 h test (LC100 = 400 ppm), dermal toxicity towards rabbits (LD50 = 4320 mg kg−1), and towards aquatic organisms like Leuciscus idus melanotus in a 48 h test (LC50 – 265 mg L−1), Daphnia in a 96 h test (EC50 = 55 mg L−1) and Desmodesmus subspicatus in a 192 h test (EC10 = 3400 mg L−1). Among other applications, acetic anhydride has been used for the in situ conversion of phenols into their corresponding acetyl derivatives.39 Similarly, trifluoroacetic anhydride (rank 59 according to the low confidence ranking) has been applied to obtain derivatives of amphetamine-type stimulants for their determination in biological samples.40 Pentafluorobenzaldehyde (rank 25 according to the low confidence ranking) has been applied to obtain aliphatic amine derivatives directly on solid phase microextraction (SPME) fibers.41N-Methyl-N-(trimethylsilyl)acetamide (high rank 7 according to both rankings) has been applied for the derivatisation of nitrophenols in hollow fibre liquid phase microextraction prior to their determination in environmental samples.42 Even though a rather green derivatisation agent is applied in a small amount (25 μL of 10 mg L−1), the main emphasis in terms of green chemistry is on the small amounts of solvent applied in the procedure. A mixture of three trimethylsilylating agents, namely 1-(trimethylsilyl)-1H-imidazole (rank 53 according to the high confidence ranking), trimethylsilyl (1E)-N-(trimethylsilyl)ethanimidate (rank 57 according to the high confidence ranking), and chloro(trimethyl)silane (rank 64 according to the high confidence ranking), has been applied for the derivatisation of polycyclic aromatic hydrocarbon quinines.43 The overall greenness of the analytical procedure was assessed with the analytical eco-scale giving positive results. Our assessment shows that these three derivatisation agents are rather problematic among those used in GC.
In another work, 1-(bromomethyl)-2,3,4,5,6-pentafluorobenzene (rank 32 according to the high confidence ranking), 1,1,1-trimethyl-N-(trimethylsilyl)silanamine (rank 69 according to the high confidence ranking), 2,2,2-trifluoro-N,N-bis(trimethylsilyl)acetamide (rank 49 according to the high confidence ranking), N-[dimethyl(2-methyl-2-propanyl)silyl]-2,2,2-trifluoro-N-methylacetamide (rank 36 according to the high confidence ranking), and acetic anhydride (rank 68 according to the high confidence ranking) were investigated in terms of applicability as SPME on-fibre derivatisation agents for phenol determination in occupational air.44 The best analytical performance was achieved with acetic anhydride, an agent that was ranked low in this assessment. The agent of the best rank here, 1-(bromomethyl)-2,3,4,5,6-pentafluorobenzene (rank 32 according to the high confidence ranking), was characterised by good analytical performance in the discussed study.
A number of GC derivatisation agents have been identified as restricted and priority substances in SUBSPORT, the SIN List, and/or the EPA's TRI chemicals list. Specifically, two silylating reagents, namely hexamethyl disiloxane and chloro(trimethyl)silane, three acylating agents, namely heptafluorobutanoic acid, propanoic anhydride and acetic anhydride, and eight alkylation/esterification agents, namely sodium methanoate, propanoic anhydride, 2-bromopropane, trichloroacetyl chloride, trichloroborane, trifluoroborane, sulphuric acid solution and dimethyl sulphate have been ranked, in general, within the third tertile of both high and low confidence rankings with the exception of heptafluorobutanoic acid, a fluorinated substance restricted in textile production.30
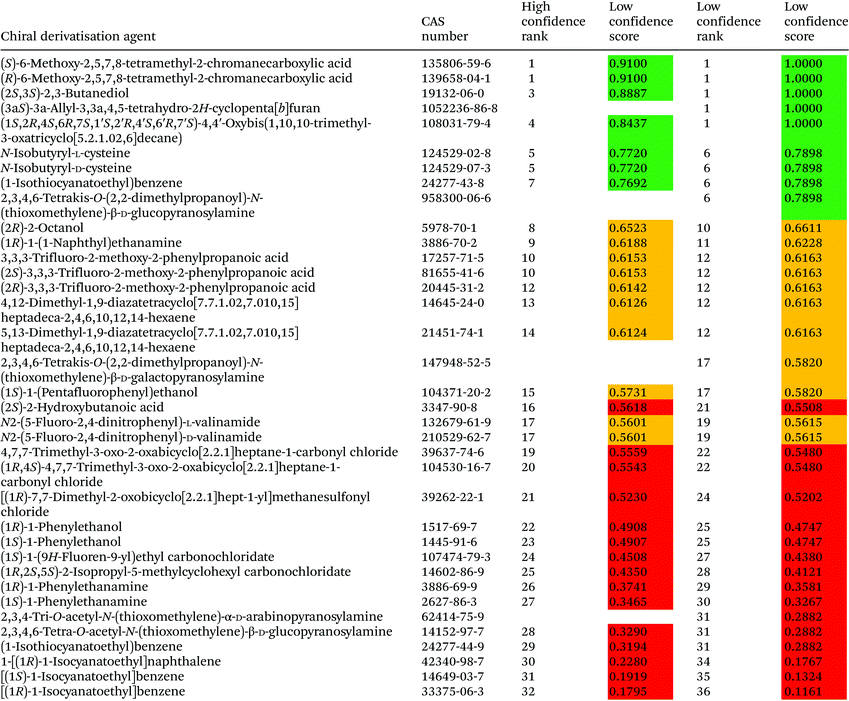
3.3. Chiral derivatisation agents assessment
It can be inferred from the basic statistics analysis of transformed hazard and precautionary statements, signal wordings and degradation products that chiral derivatisation agents are, in general, less problematic than LC and GC derivatisation agents. The mean value of hazard statements for chiral derivatisation agents is 8.7 (LC = 10.3 and GC = 13.6), precautionary statements is 20.6 (LC = 21 and GC = 31.9), signal wording is 2.08 (LC = 2.02 and GC = 3.04) and degradation/fire products is 17.3 (LC = 19.3 and GC = 18.0). None of the chiral agents is listed as a carcinogen in the IARC lists. It should be noted, however, that the assessment criteria applied here do not include all parameters that are dependent on the chirality, i.e., toxicity towards different organisms or teratogenicity,45 and, therefore, they have not been included during ranking procedures as these compounds are still weakly characterised.Among chiral derivatisation agents, the (R)- and (S)-forms of 6-methoxy-2,5,7,8-tetramethyl-2-chromanecarboxylic acid, (2S,3S)-2,3-butanediol, (1S,2R,4S,6R,7S,1′S,2′R,4′S,6′R,7′S)-4,4′-oxybis(1,10,10-trimethyl-3-oxatricyclo[5.2.1.02,6]decane), and (3aS)-3a-allyl-3,3a,4,5-tetrahydro-2H-cyclopenta[b]furan obtained the highest scores with both high and low confidence. The most problematic chiral derivatisation agents were found to be benzene and naphthalene derivatives. But even these compounds are not labelled with “fatal” effects hazard statements. (1S)-1-Phenylethanamine (rank 27 according to the high confidence ranking) is rather well characterized in terms of toxicity – oral toxicity for rats (LD50 = 950 mg kg−1), and dermal toxicity towards rabbits (LD50 = 730 mg kg−1) and to Pimephales promelas fish in a 96 h test (LC50 = 17 mg L−1). In general, isocyanate and isothiocyanate reagents were ranked low. In fact, five of them have received the lowest scores, namely 2,3,4,6-tetra-O-acetyl-N-(thioxomethylene)-β-D-glucopyranosylamine, (1-isothiocyanatoethyl)benzene, 1-[(1R)-1-isocyanatoethyl]naphthalene, [(1S)-1-isocyanatoethyl]benzene and [(1R)-1-isocyanatoethyl]benzene. None of the chiral derivatisation agents have been identified as restricted and priority substances in SUBSPORT, the SIN List, and/or the EPA's TRI chemicals list.
4. Conclusions
The present study provides an assessment, in terms of greenness, of 267 LC, GC and chiral derivatisation agents typically used in analytical chemistry and related fields. The preference rankings were performed for each group of derivatisation agents by means of MCDA according to the best relevant criteria that are available. In all three cases, fine rankings were obtained for high and low confidence assumptions.For more informative assessment, it would be beneficial to include toxicological endpoints and more information about environmental persistence among the assessment criteria. Incorporating valuable greenness indicators of synthesis processes such as the carbon footprint or energy needs during the production of each chemical as assessment criteria would be worthwhile. Unfortunately, these values are not easily available in the literature for a satisfactory number of derivatisation agents. Furthermore, the recovery of derivatisation agents is another important issue that influences the greenness of derivatisation reactions, so its inclusion as an assessment criterion would also be desirable. However, it is dependent on reaction specific conditions, not only the kind of derivatisation agent matters, but also the analytes to be determined and solvents employed.
The greenness of derivatisation agents is very rarely considered during analytical method development. The main criteria for the selection of derivatisation agents are their rapidity and efficiency, but the greenness should also be considered. This study allows selecting less problematic derivatisation agents for analytical method development while some clues can also be deduced for other than analytical applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
F. Pena-Pereira thanks Xunta de Galicia for financial support as a post-doctoral researcher of the I2C program.Notes and references
- P. T. Anastas and J. C. Warner, Green chemistry: Theory and practice, Oxford Univerity Press, New York, 1998 Search PubMed.
- M. Koel, Green Chem., 2016, 18, 923–931 RSC.
- M. de la Guardia and S. Armenta, in Green Analytical Chemistry: Theory & Practice, ed. M. de la Guardia and S. Armenta, Elsevier, Amsterdam, 2011, pp. 39–57 Search PubMed.
- C. J. Welch, N. Wu, M. Biba, R. Hartman, T. Brkovic, X. Gong, R. Helmy, W. Schafer, J. Cuff, Z. Pirzada and L. Zhou, TrAC, Trends Anal. Chem., 2010, 29, 667–680 CrossRef CAS.
- M. Koel and M. Kaljurand, Crit. Rev. Anal. Chem., 2012, 42, 192–195 CrossRef CAS.
- A. D. Curzons, D. C. Constable and V. L. Cunningham, Clean Technol. Environ. Policy, 1999, 1, 82–90 CrossRef.
- C. Jiménez-González, A. D. Curzons, D. J. C. Constable and V. L. Cunningham, Clean Technol. Environ. Policy, 2005, 7, 42–50 CrossRef.
- K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Nagy, D. A. Perry and M. Stefaniak, Green Chem., 2008, 10, 31–36 RSC.
- R. K. Henderson, C. Jiménez-González, D. J. C. Constable, S. R. Alston, G. G. A. Inglis, G. Fisher, J. Sherwood, S. P. Binks and A. D. Curzons, Green Chem., 2011, 13, 854–862 RSC.
- L. Moity, M. Durand, A. Benazzouz, C. Pierlot, V. Molinier and J.-M. Aubry, Green Chem., 2012, 14, 1132–1145 RSC.
- D. Prat, O. Pardigon, H.-W. Flemming, S. Letestu, V. Ducandas, P. Isnard, E. Guntrum, T. Senac, S. Ruisseau, P. Cruciani and P. Hosek, Org. Process Res. Dev., 2013, 17, 1517–1525 CrossRef CAS.
- M. Tobiszewski, S. Tsakovski, V. Simeonov and F. Pena-Pereira, Green Chem., 2015, 17, 4773–4785 RSC.
- M. Tobiszewski, J. Namieśnik and F. Pena-Pereira, Green Chem., 2017, 19, 1034–1042 RSC.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. Mcelroy, S. Abou-shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Nagy, D. A. Perry and M. Stefaniak, Green Chem., 2008, 10, 31–36 RSC.
- J. P. Adams, C. M. Alder, I. Andrews, A. M. Bullion, M. Campbell-Crawford, M. G. Darcy, J. D. Hayler, R. K. Henderson, C. A. Oare, I. Pendrak, A. M. Redman, L. E. Shuster, H. F. Sneddon and M. D. Walker, Green Chem., 2013, 15, 1542–1549 RSC.
- R. K. Henderson, A. P. Hill, A. M. Redman and H. F. Sneddon, Green Chem., 2015, 17, 945–949 RSC.
- M. Cinelli, S. R. Coles and K. Kirwan, Ecol. Indic., 2014, 46, 138–148 CrossRef.
- I. B. Huang, J. Keisler and I. Linkov, Sci. Total Environ., 2011, 409, 3578–3594 CrossRef CAS PubMed.
- P. Limleamthong, M. Gonzalez-Miquel, S. Papadokonstantakis, A. I. Papadopoulos, P. Seferlis and G. Guillén-Gosálbez, Green Chem., 2016, 18, 6468–6481 RSC.
- K. A. Phillips, J. F. Wambaugh, C. M. Grulke, K. L. Dionisio and K. K. Isaacs, Green Chem., 2017, 19, 1063–1074 RSC.
- D. R. Knapp, Handbook of analytical derivatization reactions, Wiley, New York, USA, 1979 Search PubMed.
- D. R. Parkinson, in Comprehensive sampling and sample preparation, ed. J. Pawliszyn and H. L. Lord, Elsevier, Academic Press, Oxford, UK, 2012, pp. 559–595 Search PubMed.
- J. Rosenfeld, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2011, 879, 1157–1158 CrossRef CAS PubMed.
- ChemSpider, http://www.chemspider.com (accessed 7 Sep 2017).
- C. R. McElroy, A. Constantinou, L. C. Jones, L. Summerton and J. H. Clark, Green Chem., 2015, 17, 3111–3121 RSC.
- A. Gałuszka, Z. M. Migaszewski, P. Konieczka and J. Namieśnik, TrAC, Trends Anal. Chem., 2012, 37, 61–72 CrossRef.
- G. A. Burdock, Encyclopedia of Food & Color Additives, CRC Press, Boca Ratón, Fl, 1st edn, 1996 Search PubMed.
- F. M. A. Rind, M. Y. Khuhawar and A. D. Rajper, J. Pharm. Biomed. Anal., 2001, 26, 331–336 CrossRef CAS PubMed.
- Subsport, http://www.subsport.eu/listoflists (accessed 7 Sep 2017).
- SINList, http://sinlist.chemsec.org/ (accessed 7 Sep 2017).
- EPA, https://www.epa.gov/toxics-release-inventory-tri-program/tri-listed-chemicals (accessed 2 Oct 2017).
- P. Miralles, I. Vrouvaki, A. Chisvert and A. Salvador, Talanta, 2016, 154, 1–6 CrossRef CAS PubMed.
- S. Fanali, P. R. Haddad, C. Poole and M.-L. Riekkola, Liquid chromatography - Applications, Elsevier, 2nd edn, 2017 Search PubMed.
- C. K. Zacharis and P. D. Tzanavaras, Anal. Chim. Acta, 2013, 798, 1–24 CrossRef CAS PubMed.
- J. M. Płotka-Wasylka, C. Morrison, M. Biziuk and J. J. Namieśnik, Chem. Rev., 2015, 115, 4693–4718 CrossRef PubMed.
- A. Escrig-Doménech, E. F. Simó-Alfonso, J. M. Herrero-Martínez and G. Ramis-Ramos, J. Chromatogr., A, 2013, 1296, 140–156 CrossRef PubMed.
- B. Qi, P. Liu, Q. Wang, W. Cai, B. Yuan and Y. Feng, Trends Anal. Chem., 2014, 59, 121–132 CrossRef CAS.
- J. Olejniczak and J. Staniewski, Anal. Chim. Acta, 2007, 588, 64–72 CrossRef CAS PubMed.
- M. K. Woźniak, M. Wiergowski, J. Aszyk, P. Kubica, J. Namieśnik and M. Biziuk, J. Pharm. Biomed. Anal., 2017, 148, 58–64 CrossRef PubMed.
- E. Gionfriddo, A. Passarini and J. Pawliszyn, J. Chromatogr., A, 2016, 1457, 22–28 CrossRef CAS PubMed.
- H. R. Sobhi, A. Esrafili, H. Farahani, M. Gholami and M. M. Baneshi, Environ. Monit. Assess., 2013, 185, 9055–9065 CrossRef CAS PubMed.
- A. Toriba, C. Homma, M. Kita, W. Uozaki, Y. Boongla, W. Orakij, N. Tang, T. Kameda and K. Hayakawa, J. Chromatogr., A, 2016, 1459, 89–100 CrossRef CAS PubMed.
- A. Es-haghi, M. Baghernejad and H. Bagheri, Anal. Chim. Acta, 2012, 742, 17–21 CrossRef CAS PubMed.
- S. W. Smith, Toxicol. Sci., 2009, 110, 4–30 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Original dataset. See DOI: 10.1039/c7gc03108d |
| This journal is © The Royal Society of Chemistry 2017 |