Mid-chain carboxylic acids by catalytic refining of microalgae oil†
Abstract
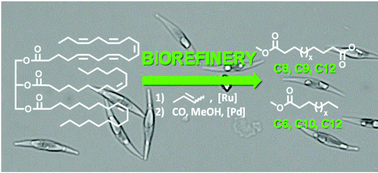
Microalgae oil serves as a feedstock for a biorefinery approach to mid-chain (di-)carboxylic acid esters, currently only accessible via demanding synthetic routes. Via the butenolysis of mono- and poly-unsaturated fatty acids, short-chain unsaturated fatty acid methyl esters and mono- and di-enes were produced in a high selectivity. These olefins were further processed into value added linear mid-chain (di-)carboxylic acid esters via isomerizing alkoxycarbonylation. Model compounds such as eicosapentaenoic acid were used to study the reactions including the screening of metathesis catalysts and to identify all formed products. Notably, eicosapentaenoic acid, a five-fold unsaturated fatty acid relatively abundant in algae, is successfully converted to four equivalents of heptadiene, which was carbonylated to the linear diester (dimethyl azelate). The butenolysis and subsequent isomerizing alkoxycarbonylation were performed on the algae oil extracted from the diatom Phaeodactylum tricornutum. Despite the multicomponent mixture of numerous lipids and non-lipid compounds present in algae oil, high conversion and high selectivity for the desired products were achieved in both reactions. This approach provides access to several carboxylic mono- and di-acid esters of chain length ranging from C6 to C12 (amongst others azelaic acid ester, suberic acid ester and dodecanedioic acid ester), that are in demand but to which access is limited currently, fully based on algae oils as a renewable resource.


 Please wait while we load your content...
Please wait while we load your content...