Solvent-free implementation of two dissimilar reactions using recyclable CuO nanoparticles under ball-milling conditions: synthesis of new oxindole-triazole pharmacophores†
Abstract
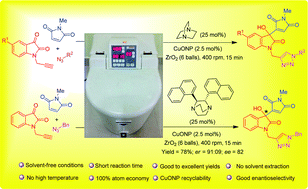
Herein, synthesis of new hybrid pharmocophores by merging Baylis–Hillman and click chemistry under ball-milling conditions was accomplished. The substrate scope of the reaction was broadened to include alkyl, benzyl, and aryl groups. Catalytic amount of CuO nanoparticles (CuONPs < 50 nm), which was used to carry out the click reaction, could be easily recovered by simple centrifugation, and CuONPs were found to be catalytically active for almost 6 cycles. The highlights of the proposed protocol are as follows: (i) solvent-free reaction conditions, (ii) short reaction times, (iii) no rigorous solvent extraction, (iv) good to excellent chemical yields, (v) 100% atom economy, (vi) CuONP recyclability, (vii) good enantioselectivity, and (viii) no need for high temperatures. Overall, the developed protocol significantly broadens the scope of mechanochemistry as it involves two dissimilar reactions with equal aplomb in a sustainable fashion. Biological assays and in silico studies of the synthesized compounds were also performed to showcase the efficacy of the synthesized compounds as antimicrobial agents.


 Please wait while we load your content...
Please wait while we load your content...