Open Access Article
Open Access ArticleGrinding-induced functionalization of carbon-encapsulated iron nanoparticles†
Artur
Kasprzak
 *a,
Michał
Bystrzejewski
b,
Mariola
Koszytkowska-Stawinska
a and
Magdalena
Poplawska
a
*a,
Michał
Bystrzejewski
b,
Mariola
Koszytkowska-Stawinska
a and
Magdalena
Poplawska
a
aFaculty of Chemistry, Warsaw University of Technology, 00-664 Warsaw, Poland. E-mail: akasprzak@ch.pw.edu.pl
bDepartment of Chemistry, University of Warsaw, 02-093 Warsaw, Poland
First published on 5th May 2017
Abstract
Covalent functionalization of carbon-encapsulated iron nanoparticles based on the grinding-induced 1,3-cycloaddition reaction of nitrile oxides is presented. We report an easy to perform, fast and efficient method for the direct introduction of various types of functional moieties, such as carboxylic, metallocene and sugar units, into carbon-encapsulated iron nanoparticles.
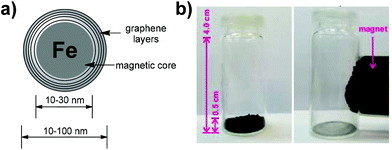
Carbon-encapsulated iron nanoparticles (CEINs) are a class of carbon-based nanomaterials which possess an encouraging wide-range of possible applications, including medicinal chemistry,1 adsorption technologies2 and catalysis.3 This broad scope of application is directly associated with their physical and chemical properties, such as core–shell morphology (a ferromagnetic core covered by curved graphene layers; Fig. 1a), as well as strong magnetic properties (Fig. 1b). Nevertheless, all the pristine carbon-based nanomaterials need to be functionalized before the synthesis of more complex constructs, because of the lack of functional groups on their surface. Most commonly, the introduction of organic moieties onto various carbon nanomaterials, if possible, is associated with the use of (i) significant amounts of toxic solvents, like 1,2-dichlorobenzene, toluene or N,N-dimethylformamide, (ii) high temperature and long-time reactions, as well as (iii) stepwise protocols, e.g. deprotection reactions.4 With the increasing number of articles on the application of carbon nanomaterials in various types of functional materials the above-mentioned phenomenon can be regarded as highly environmentally unfriendly.
In the last ten years the mechanochemistry has attracted an increasing attention of scientists because of its versatility to create various types of covalent bonds between chemical individuals.5 For example, it has been found that such a green and simple method can be applied to obtain (i) graphene nanosheets via mechanochemical exfoliation,6a and (ii) graphene-based hybrid materials.6b,c To date, many reactions which are specific for carbon materials, especially fullerenes, have been reported to proceed under grinding-induced conditions.7 For example, the direct introduction of hydroxyl groups onto fullerenes (synthesis of fulleroles), carbon nanotubes and graphene via ball milling with potassium hydroxide for a relatively long time (from 30 min up to 9 h) has been demonstrated. Cycloaddition reactions between fullerenes and nitrile oxides, which can be derived via oxidation of oximes, are also possible to proceed under grinding-induced conditions.8 This type of mechanochemical reaction can be regarded as one of the most versatile and the most efficient techniques to introduce various functional moieties onto the surface of carbon nanomaterials. However, the cycloaddition reaction, if possible, is most commonly conducted as a solution-phase functionalization with the use of significant amounts of toxic solvents and long-time high-temperature reactions.4a,9 What is more important, there are no examples on the cycloaddition reaction between carbon nanomaterials and some specific moieties, e.g. unprotected carboxyl-containing compounds or unprotected sugars.
Herein we report, a novel mechanochemical, eco-friendly and grinding-induced method for the functionalization of carbon-encapsulated iron nanoparticles (Scheme 1). Our method employs the 1,3-cycloaddition reaction of nitrile oxides bearing unprotected carboxyl or hydroxyl moieties, as well as a metalorganic or a sugar unit. To the best of our knowledge there are no examples on such mechanochemical introduction of the organic moieties onto a graphene-based material. Such conclusion prompted us to develop a simple, efficient, non-toxic and fast method to functionalize this unique magnetic nanomaterial. We have carefully selected the aldehydes containing different types of specific functionalities to show the simplicity and effectiveness of mechanochemical introduction of the organic moieties into the surface of carbon-encapsulated iron nanoparticles.
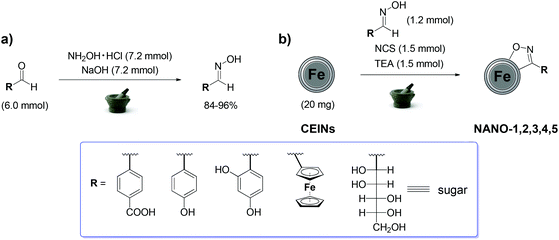 | ||
| Scheme 1 The developed grinding-induced and eco-friendly mechanochemical processes: synthesis of the oximes (a) and functionalization of CEINs (b). | ||
Experimental details are presented in the ESI.† The oximes were obtained via a mechanochemical reaction between the appropriate aldehyde (6.0 mmol), hydroxylamine hydrochloride (7.2 mmol) and solid sodium hydroxide (7.2 mmol) (Scheme 1a). We have modified the purification method in a similar mechanochemical process presented earlier (for 4-carboxybenzaldoxime and hydroxybenzaldoximes),10 which resulted in the improvement of the process yield (ca. 84–96%). Moreover, we have broadened the scope of the aldehydes, i.e. ferrocene based-oxime and sugar oxime were also included in this study. NMR spectroscopy (see Fig. S1–S10 in the ESI†) confirmed the formation of a pure product and full conversion of the aldehyde into the oxime in each case. E-Oximes were obtained based on the literature data (see details in the ESI†).
CEINs used in this study were obtained via a carbon arc discharge route and they have the diameter between 10–100 nm, whilst the carbon coating is formulated of ca. 5–20 curved graphene layers (Fig. 1a).11 The functionalization of CEINs was based on the grinding-induced and eco-friendly mechanochemical cycloaddition reaction of nitrile oxides generated from the as-obtained oximes (Scheme 1b). Oxime (1.2 mmol), N-chlorosuccinimide (1.5 mmol) and CEINs (20 mg) were placed in an agate mortar and ground together with an agate pestle for 1 minute followed by the addition of a small amount of triethylamine (140 μL; 1.5 mmol) and pyridine (20 μL; 0.2 mmol). Next, the mixture was ground for 8 min at room temperature. The carbon material was sonicated with an appropriate solvent for 2 hours, filtered off and dried at 45 °C overnight.
The mass gain was observed for each resulting carbon nanomaterial (see Experimental details in the ESI†). Nevertheless, this fact only partially indicates the success of the functionalization because of highly hygroscopic features of the product. Hence, the progress of functionalization was tracked qualitatively by Fourier-transform infrared (FT-IR) spectroscopy, X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM) and quantitatively by thermogravimetry (TGA).
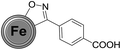
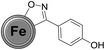
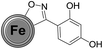
Firstly, the success of the grinding-induced mechanochemical functionalization of CEINs was analysed qualitatively. The representative FT-IR spectrum of CEINs containing a 3-(4-carboxyphenyl)isoxazoline moiety (NANO-1) is presented in Fig. 2 (the FT-IR spectra for other products are shown in Fig. S16–S19 in the ESI†). For comparison, the spectra of pristine CEINs and the oximes are also shown. The absorption bands typical of the isoxazoline-modified carbon nanomaterial as well as the bands characteristic of the given oxime were observed in the spectrum of each product. For example, the most prominent bands for NANO-1 are at 1685, 1610, 1415, 1285, 1085, 860, 760, 730, 685 and 540 cm−1. Almost the same bands as for the raw oxime are observed in the spectrum of the nanomaterial, which is related to the presence of the isoxazoline ring on the nanomaterial (several types of stretching vibrations coming from N–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N moieties). The bands at 1610, 860, 685 and 540 cm−1 correspond to the benzene ring stretching (1610 cm−1) and benzene ring deformation vibrations (for other bands; including out-of-plane vibrations). Please note that for NANO-1 (reaction with 4-carboxybenzaldoxime) the typical absorption band at ca. 1685 cm−1, associated with the presence of the carboxyl group, can be found in the spectrum of the isoxazoline-modified carbon material. If so, one can conclude that the developed mechanochemical protocol (i) yields the desired product bearing the unprotected carboxyl group on the surface of CEINs, as well as (ii) does not induce any side reaction with the inclusion of the COOH group. To the best of our knowledge there are no examples of such direct cycloaddition-based introduction of the organic compound containing the unprotected carboxyl group onto carbon nanomaterials. The same conclusion is found for CEINs modified with the unprotected sugar moiety (NANO-5). The ferrocene moiety was also introduced into CEINs (product NANO-4) via the herein presented mechanochemical route. All the discussed observations are the undoubted evidence of the versatility and potential of the developed mechanochemical process.
N moieties). The bands at 1610, 860, 685 and 540 cm−1 correspond to the benzene ring stretching (1610 cm−1) and benzene ring deformation vibrations (for other bands; including out-of-plane vibrations). Please note that for NANO-1 (reaction with 4-carboxybenzaldoxime) the typical absorption band at ca. 1685 cm−1, associated with the presence of the carboxyl group, can be found in the spectrum of the isoxazoline-modified carbon material. If so, one can conclude that the developed mechanochemical protocol (i) yields the desired product bearing the unprotected carboxyl group on the surface of CEINs, as well as (ii) does not induce any side reaction with the inclusion of the COOH group. To the best of our knowledge there are no examples of such direct cycloaddition-based introduction of the organic compound containing the unprotected carboxyl group onto carbon nanomaterials. The same conclusion is found for CEINs modified with the unprotected sugar moiety (NANO-5). The ferrocene moiety was also introduced into CEINs (product NANO-4) via the herein presented mechanochemical route. All the discussed observations are the undoubted evidence of the versatility and potential of the developed mechanochemical process.
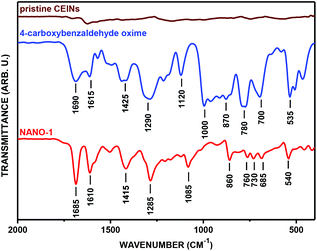 | ||
| Fig. 2 FT-IR spectrum of NANO-1. See Table 1 for legends. | ||
Thermogravimetry (TGA) was applied for the quantitative evaluation of the functionalization yield. The representative TGA curve (nitrogen atmosphere) of CEINs containing the 3-(4-carboxyphenyl)isoxazoline moiety (NANO-1) is presented in Fig. 3 (the TGA curves for other products are presented in Fig. S20–S24 in the ESI†). For comparison, the TGA curve of pristine CEINs is also shown. The first weight loss, between ca. 65 °C–120 °C, is related to the presence of moisture in the sample. Further weight loss (which is not observed for pristine CEINs) starts at ca. 150 °C and is completed at ca. 550 °C, which is clearly attributed to the decomposition of covalently attached moieties.9c,12 The weight loss for each sample is an indirect indicator of the content of the introduced organic moiety. The calculated content of the introduced moiety for each sample is 16–33 wt% (see data in Table 1). The details of the calculation of the content of the introduced moiety are shown in the ESI (section 4).†
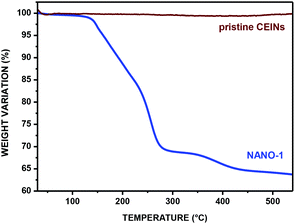 | ||
| Fig. 3 TGA curve (in nitrogen) of NANO-1. See Table 1 for legends. | ||
X-Ray photoelectron spectroscopy (XPS) analyses were also performed to confirm the successful covalent functionalization of CEINs. The survey spectra of all materials are presented in Fig. S25–S30 in the ESI.† The survey spectra reveal the presence of three major components, namely carbon, iron and oxygen. The presence of nitrogen was observed in NANO-1–NANO-5 samples, only. This finding directly proves that the functionalization was successful. The proposed cycloaddition route (Scheme 1) was finally confirmed via the analysis of the deconvoluted C 1s component (Fig. 4). The C 1s component of pristine CEINs (Fig. 4a) contains one peak, which is centered at 284.6 eV and corresponds to sp2 carbons. The deconvoluted C 1s spectra for the functionalized samples are comprised of at least two peaks, located at 284.5 eV (sp2 carbons) and 285.6–286.5 eV. The latter feature can be assigned to C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N moieties, and in fact, this observation confirms the proposed reaction scheme. Additionally, the C 1s of NANO-1 (Fig. 4b) contains an additional peak at 289.1 eV, which is typical of carboxylic groups (the presence of the COOH group was also evidenced in the FT-IR spectrum, Fig. 2). Obviously, the attached molecule in NANO-1 contains the carboxylic group in its structure.
N moieties, and in fact, this observation confirms the proposed reaction scheme. Additionally, the C 1s of NANO-1 (Fig. 4b) contains an additional peak at 289.1 eV, which is typical of carboxylic groups (the presence of the COOH group was also evidenced in the FT-IR spectrum, Fig. 2). Obviously, the attached molecule in NANO-1 contains the carboxylic group in its structure.
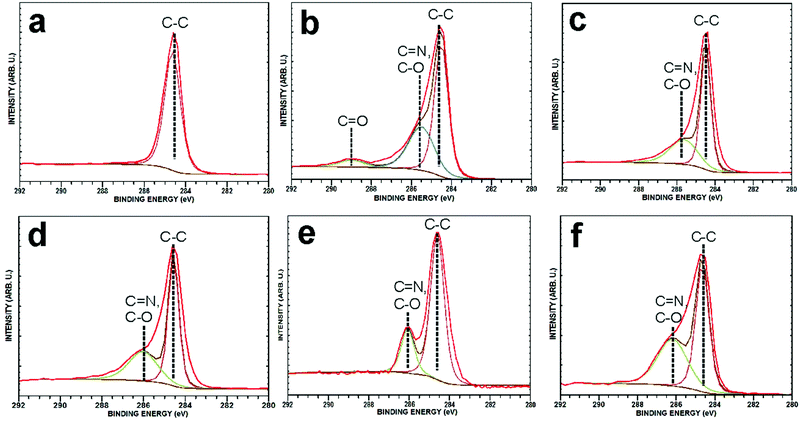 | ||
| Fig. 4 XPS curve fitting of C 1s peaks of pristine CEINs (a), NANO-1 (b), NANO-2 (c), NANO-3 (d), NANO-4 (e) and NANO-5 (f). | ||
The transmission electron microscopy (TEM) studies were also performed to visualize any morphological changes on the surface of functionalized CEINs. The representative TEM image of NANO-1 is shown in Fig. 5 (the TEM images of other materials are presented in Fig. S31–S34 in the ESI†). The TEM image of pristine CEINs is presented elsewhere.11 The surface modification of CEINs is clearly seen. After functionalization the nanomaterial is covered by an inhomogeneous, very thin and nonuniform layer, which corresponds to the presence of moieties covalently attached to CEINs.
To exclude the possibility of physical adsorption of the aromatic moieties onto CEINs, the examined aromatic oximes were ground with CEINs for 8 minutes in the absence of the reactants needed for the generation of nitrile oxides (N-chlorosuccinimide and triethylamine). There were no weight gain, no characteristic signals for the organic moieties in the FT-IR spectra and no essential weight loss in the TGA curves for the resulting carbon materials. If so, one can conclude that the studied oximes are not permanently adsorbed onto the CEINs in the course of the desired protocol. This finding supports our thesis of a covalent functionalization of CEINs using the environmentally improved and grinding-induced method. Moreover, we have additionally performed some attempts to functionalize CEINs using the traditional liquid-phase reaction recently reported by Boncel et al.4a (for functionalization of carbon nanotubes with e.g. 4-hydroxybenzaldoxime). We have selected 4-carboxybenzaldoxime, 4-hydroxybenzaldoxime and ferrocenecarboxaldehyde oxime to be attached to the CEIN surface using the above-mentioned method. Once again, FT-IR analysis has not indicated any reaction (the lack of characteristic absorptions bands).
Finally, water dispersions (with high concentration of 500 μg mL−1) of the representative NANO-1 and NANO-5 were prepared to examine the colloidal stability of the carboxy- or sugar-modified CEINs. As it can be seen in Fig. 6, water dispersions of the CEINs containing the 3-(4-carboxyphenyl)isoxazoline moiety (NANO-1) and the 3-(D-mannosyl)isoxazoline moiety (NANO-5) exhibit incomparable higher colloidal stability in comparison to the pristine CEINs. Such observation finally indicates the success of surface functionalization and is very important in terms of possible bio-application of such constructs.
 | ||
| Fig. 6 Water dispersions (500 μg mL−1) of NANO-1 and NANO-5, 10 min after sonication. Water dispersion of pristine CEINs is also presented. | ||
Conclusions
An efficient, eco-friendly and grinding-induced mechanochemical covalent functionalization of the carbon-encapsulated iron nanoparticles has been developed. It has been demonstrated that it is possible to conduct the cycloaddition reactions using magnetic carbon-based nanomaterials and the unprotected oximes, like carboxy- and hydroxy-containing compounds (sugar oximes can also be used), as well as metallocene oximes, such as ferrocenecarboxyaldehyde oxime. The presented route is fast (reaction time 8 minutes), versatile and does not require the use of toxic solvents and provides high functionalization yields. The observed contents of introduced moieties (16–33 wt%) using such an environmentally improved protocol are nearly the same or even higher in comparison to the typical liquid-phase functionalization of CEINs and other carbon nanomaterials, using e.g. 4-(dimethylamino)benzaldehyde oxime (ca. 22 wt%).9cAcknowledgements
This work was financially supported partially by the National Science Center (Poland) through the grant PRELUDIUM (No. 2016/21/N/ST5/00864) “Nanotheranostics dedicated to targeted anticancer therapies: Novel magnetic hybrid carbon-based materials – synthesis and characterization” and the Warsaw University of Technology. Artur Kasprzak thanks the Warsaw Consortium of Academic Chemistry for KNOW scholarship. The XPS studies were carried out at the Biological and Chemical Research Centre, University of Warsaw, established within the project co-financed by the European Union from the European Regional Development Fund under the Operational Programme Innovative Economy, 2007–2013. The authors appreciate the help from Prof. Adam Lewera with X-Ray Photoelectron Spectroscopy experiments.References
- (a) G. Modugno, C. Menard-Moyon, M. Prato and A. Bianco, Br. J. Pharmacol., 2015, 172, 975–991 CrossRef CAS PubMed; (b) M. A. Cywinska, M. Bystrzejewski, M. Poplawska, A. Kosmider, R. Zdanowski, S. Lewicki, Z. Fijalek, A. Ostrowska, M. Bamburowicz, A. Cieszanowski and I. P. Grudzinski, Toxicol. In Vitro, 2016, 34, 229–236 CrossRef CAS PubMed.
- (a) M. Bystrzejewski, K. Pyrzynska, A. Huczko and H. Lange, Carbon, 2009, 47, 1201–1204 CrossRef CAS; (b) H. Niu, Y. Wang, X. Zhang, Z. Meng and Y. Cai, ACS Appl. Mater. Interfaces, 2012, 4, 286–295 CrossRef CAS PubMed.
- J. A. Varnell, E. C. M. Tse, C. E. Schultz, T. T. Fister, R. C. Haash, J. Timoshenko, A. I. Frenkel and A. A. Gewirth, Nat. Commun., 2016, 7, 12582 CrossRef CAS PubMed.
- (a) A. P. Herman and S. Boncel, RSC Adv., 2016, 6, 64129 RSC; (b) H.-T. Yang, X.-J. Ruan, C.-B. Miao and X.-Q. Sun, Tetrahedron Lett., 2010, 51, 6056–6059 CrossRef CAS; (c) T.-X. Liu, J. Ma, D. Chao, P. Zhang, Q. Liu, L. Shi, Z. Zhang and G. Zhang, Chem. Commun., 2015, 51, 12775 RSC; (d) H. Kato, A. Yashiro, A. Mizuno, Y. Nishida, K. Kobayashi and H. Shinohara, Bioorg. Med. Chem. Lett., 2001, 11, 2935–2939 CrossRef CAS PubMed.
- (a) G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668 RSC; (b) S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friscic, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC; (c) See a web themed issue ‘Mechanochemistry: fundamentals and applications in synthesis’ in Chem. Commun: S. L. James and T. Friscic, Chem. Commun., 2013, 49, 5349–5350 RSC.
- (a) V. Leon, J. M. Gonzalez-Dominguez, J. L. G. Fierro, M. Prato and E. Vazquez, Nanoscale, 2016, 8, 14548 RSC; (b) K.-C. Mei, Y. Guo, J. Bai, P. M. Costa, H. Kafa, A. Protti, R. C. Hider and K. T. Al-Jamal, ACS Appl. Mater. Interfaces, 2015, 7, 14176–14181 CrossRef CAS PubMed; (c) N. Rubio, K.-C. Mei, R. Klippstein, P. M. Costa, N. Hodgins, J. Tzu-Wen Wang, F. Festy, V. Abbate, R. C. Hider, K. Lung Andrew Chan and K. T. Al-Jamal, ACS Appl. Mater. Interfaces, 2015, 7, 18920–18923 CrossRef CAS PubMed.
- S.-E. Zhu, F. Li and G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7535 RSC.
- (a) J. Safaei-Ghomi and R. Masoomi, Chem. Heterocycl. Compd., 2015, 51, 39–43 CrossRef CAS; (b) J. Safaei-Ghomi and R. Masoomi, Ultrason. Sonochem., 2015, 23, 212–218 CrossRef CAS PubMed.
- (a) M. Alvaro, P. Atienzar, P. de la Cruz, J. L. Delgado, V. Troiani, H. Garcia, F. Langa, A. Palkar and L. Echegoyen, J. Am. Chem. Soc., 2006, 128, 6626–6635 CrossRef CAS PubMed; (b) M. Barrejon, M. J. Gomez-Escalonilla, J. L. G. Fierro, P. Prieto, J. R. Carrillo, A. M. Rodrıguez, G. Abellan, M. C. Lopez-Escalante, M. Gabas, J. T. Lopez-Navarrete and F. Langa, Phys. Chem. Chem. Phys., 2016, 18, 29582 RSC; (c) M. Poplawska, G. Z. Zukowska, S. Cudzilo and M. Bystrzejewski, Carbon, 2010, 48, 1312–1320 CrossRef.
- C. B. Aakero, A. S. Sinha, K. N. Epa, C. L. Spartz and J. Desper, Chem. Commun., 2012, 48, 11289–11291 RSC.
- (a) M. Bystrzejewski, A. Huczko and H. Lange, Sens. Actuators, B, 2005, 109, 81–85 CrossRef CAS; (b) J. Borysiuk, A. Grabias, J. Szczytko, M. Bystrzejewski, A. Twardowski and H. Lange, Carbon, 2008, 46, 1693–1701 CrossRef CAS.
- Typical temperature range of decomposition of covalently bound moieties to CEINs (see also ref. 9c): (a) A. Kasprzak, M. Poplawska, M. Bystrzejewski, O. Labedz and I. P. Grudzinski, RSC Adv., 2015, 5, 85556 RSC; (b) M. Poplawska, M. Bystrzejewski, I. P. Grudzinski, M. A. Cywinska, J. Ostapko and A. Cieszanowski, Carbon, 2014, 74, 180–194 CrossRef CAS; (c) A. Kasprzak, M. Poplawska, M. Bystrzejewski and I. P. Grudzinski, J. Mater. Chem. B, 2016, 4, 5593 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, Experimental details, spectroscopic (NMR, FT-IR, XPS) and TGA data. See DOI: 10.1039/c7gc00282c |
| This journal is © The Royal Society of Chemistry 2017 |