Quantitative chemocatalytic production of lactic acid from glucose under anaerobic conditions at room temperature†
Luyang
Li
ab,
Feng
Shen
a,
Richard L.
Smith
c and
Xinhua
Qi
*a
aAgro-Environmental Protection Institute, Chinese Academy of Agricultural Sciences, No. 31, Fukang Road, Nankai District, Tianjin 300191, China. E-mail: qixinhua@nankai.edu.cn; Tel: +86-22-2361-6651; Fax: +86-22-2361-6651
bCollege of Environmental Science and Engineering, Nankai University, No. 94, Weijin Road, Nankai District, Tianjin 300071, China
cResearch Center of Supercritical Fluid Technology, Tohoku University, 6-6-11 Aoba, Aramaki, Aoba-ku, Sendai 980-8579, Japan
First published on 20th October 2016
Abstract
Chemical conversion of glucose into lactic acid in water requires harsh reaction conditions to obtain relatively low product yields. Herein, a simple, but highly efficient chemocatalytic process is reported for the production of lactic acid from glucose in the presence of Ba(OH)2 under a nitrogen atmosphere at 1 bar total pressure, where glucose was selectively converted to lactic acid with a yield of 95.4% at room temperature in 48 h. The process was applied to cellobiose, fructose, dihydroxyacetone, glyceraldehyde, pyruvaldehyde and cellulose hydrolysate, among which pyruvaldehyde afforded ca. 100% lactic acid yield. Product distribution changed towards glyceric acid, glycolic acid, formic acid, malonic acid and CO2, by variation of the O2 partial pressure, which promotes the oxidation of glyceraldehyde and 1,3-dihydroxyacetone intermediates. The process developed has significant advantages over previous methods in aspects of efficiency, conditions, reactor materials and productivity.
Introduction
Sustainable chemistry for society provides the motivation to produce chemicals and fuels from biomass1–3 for which platform chemicals such as 5-hydroxymethylfurfural, levulinic acid, formic acid and furfural from bioresources have been widely studied.4–10 Among these chemicals, 2-hydroxypropanoic acid, which is commonly known as lactic acid, is one of the most important building-block intermediates, since it has wide application in the food, pharmaceutical, cosmetic, and chemical industries, and especially in the production of biodegradable plastics.11–13Currently, the production capacity of lactic acid is 3.3 × 105 tons per annum with more than 90% of the commercial lactic acid being produced by fermentation of sugars such as glucose and sucrose.14 Production of lactic acid by fermentation has some drawbacks such as high cost, long fermentation times (3–5 d), restricted large-scale operation and low productivity since lactic acid bacteria are pH sensitive.15 The growing demand for lactic acid necessitates the development of efficient and robust production processes. Chemocatalytic attempts to produce lactic acid or alkyl lactate from many starting materials such as cellulose, sugars, glycerol, dihydroxyacetone and glyceraldehyde in water or organic solvents have been widely applied.15–21 Although trioses, which are intermediates generated in the reaction of hexose to lactic acid via retro-aldol condensation, have been effectively used to form lactic acid or lactate with satisfactory yields,22–27 it is clear that reaction pathways that use hexoses instead of trioses to produce lactic acid would be preferable from aspects of cost and material supply. However, high-yield production of lactic acid from hexoses such as glucose and fructose as starting materials with chemocatalytic techniques has yet to be realized due to the complex reaction pathways and the formation of many undesirable byproducts that occur in the reaction systems.13
Diverse catalytic processes for the production of lactic acid from hexoses under hydrothermal conditions have been investigated in the presence of homogeneous or heterogeneous catalysts such as metal salts,28,29 zeolites,18 tungstated alumina,30 sodium silicate31 and alkali,16 but typically low or moderate lactic acid yields (10–55%) are obtained.16,28,32–34 For example, Heeres et al. examined the conversion of glucose in aqueous solution in the presence of several metal ions at 140 °C for 6 h, but lactic acid yields up to 20% were achieved with 100% glucose conversion.29 Huo et al. developed a process for the production of lactic acid from glucose in the presence of metal ions under alkaline hydrothermal conditions (300 °C), and a highest lactic acid yield of 25% could be obtained.28 Although glucose and sucrose could be converted into methyl lactate in methanol solvent catalyzed by zeotype or bifunctional carbon–silica catalysts, with the highest yields up to 43% and 64%, respectively, the reaction was conducted at 160 °C reaction temperature and 20 h reaction time.18,25
Alkalis have been widely used in the reaction for a long time, but the reaction was always performed under harsh hydrothermal conditions that low to moderate yields of 10–55% were reported.16,28,32–34 The applied high temperatures and pressures under these hydrothermal conditions not only increase the energy input and equipment investment, but also limit the scaling up of the process. Therefore, more efforts should be made to develop efficient methods for lactic acid production under mild conditions. Herein, we report an efficient process where glucose can be quantitatively converted into lactic acid with a common base catalyst, barium hydroxide, at room temperature and under anaerobic conditions. Product distribution can be controlled by simply varying the oxygen content in the atmosphere applied to the reaction. The proposed method allows efficient production of lactic acid from many different biomass-derived carbohydrates at room temperature and atmospheric pressure.
Results and discussion
Initially, a mixture of glucose (0.1 M) and NaOH aqueous solution (0.5 M) was prepared and stored at room temperature for two days, after which it was observed that 78.3% of the glucose was converted into lactic acid and fructose with yields of 40.2% and 30.0%, respectively. Unexpectedly, it was found that glucose could be transformed into lactic acid at room temperature with moderate selectivity, thus motivating further chemocatalytic studies.Several common alkali hydroxides (NaOH and KOH) and alkaline-earth metal hydroxides (Mg(OH)2 and Ba(OH)2) were selected to study the catalytic conversion of glucose in aqueous solutions at room temperature. Considering that the reaction environment affects the transformation of carbohydrates under hydrothermal conditions,25,35,36 the atmosphere of the aqueous solution was strictly controlled. Experiments were conducted at room temperature (25 °C) with 0.1 M glucose aqueous solution and catalysts having 0.5 M OH− concentration under a nitrogen atmosphere at 1 bar total pressure (Table 1) or under other atmospheres as discussed later. Both NaOH and KOH were active for glucose conversion while Mg(OH)2 was inactive (Table 1). The lack of activity of Mg(OH)2 can be explained by its low solubility in water (0.0064 g L−1 at 25 °C) and its weak basicity. On the other hand, glucose could be effectively converted by Ba(OH)2 at 25 °C for which glucose conversion was 97.3% and lactic acid yield was 78.3% for 48 h reaction time.
| Catalystb | Catalyst concentration (M) | Glucose conversion (%) | Product yield (%) | ||
|---|---|---|---|---|---|
| Fructose | Glyceraldehyde | Lactic acid | |||
| a Reaction time, 48 h; nitrogen atmosphere, 1 bar total pressure. b Reaction with BaCl2 (0.25 M) gave 0% glucose conversion. | |||||
| Mg(OH)2 | 0.25 | 3.0 | 1.2 | 0 | 0 |
| KOH | 0.5 | 75.7 | 29.9 | 2.6 | 38.8 |
| NaOH | 0.5 | 77.4 | 30.1 | 2.9 | 40.0 |
| Ba(OH)2 | 0.25 | 97.3 | 3.3 | 5.8 | 78.3 |
The catalytic activity of Ba(OH)2 for lactic acid formation was much higher than that of NaOH and KOH having the same OH− concentration (0.5 M), indicating that the divalent Ba2+ ion played an important role in promoting the transformation of glucose and its intermediates to lactic acid. When the reaction was performed in the presence of BaCl2, no glucose conversion occurred, which means that the co-existence of Ba2+ and OH− was essential to the transformation of glucose into lactic acid. Since Ba(OH)2 exhibited high activity for lactic acid formation from glucose at room temperature, further work focused on the chemocatalytic conversion of glucose in Ba(OH)2 aqueous solutions at 25 °C.
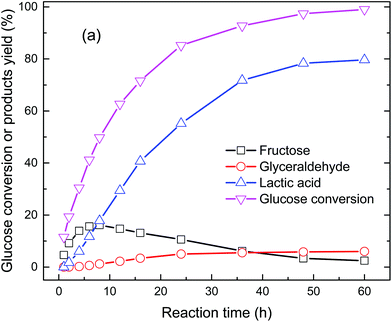
Glucose conversion as a function of reaction time catalyzed by Ba(OH)2 at room temperature and atmospheric pressure was investigated (Fig. 1 and S1, ESI†). Almost all of the glucose was converted within 60 h reaction time. At the beginning of the reaction (1 h), only fructose was detected. As the reaction proceeded, the fructose yield gradually increased and reached a peak value of 16.1% in 8 h and then gradually decreased. Small amounts of lactic acid appeared in 2 h (1.7% yield) and then steadily increased with time. A lactic acid yield of 79.7% was obtained in 60 h reaction time (Fig. 1). Glyceraldehyde (GLY), which is an intermediate in fructose fragmentation by retro-aldol reaction, formed at yields up to 6%. However, other C3 intermediates, dihydroxyacetone (DHA) and pyruvaldehyde were not detected in the products, which maybe the result of their instantaneous transformation into dimeric DHA and lactic acid, respectively, once they formed in the reaction system.26
Different sugars were used as substrates to examine their applicability for the production of lactic acid in the same catalytic system (Table 2). It shows that DHA and pyruvaldehyde afforded lactic acid yields of 87% and 99.8%, respectively, with almost complete feedstock conversion. In contrast, GLY gave a lactic acid yield of 53.1% for a GLY conversion of 93% in 48 h (Table 2). The yield of lactic acid starting from fructose was somewhat higher than that starting from glucose (83.5% versus 78.3%), which can be attributed to fructose being directly transformed into DHA and GLY by retro-aldol reaction without the isomerization step that is required for glucose. Cellobiose could be efficiently converted into lactic acid with a yield of 38.9% being obtained, although glucose, fructose, GLY and humins were obtained as by-products. Avicel (DPV 219) and ball-milling cellulose (500 rpm, 4 h) were stable in the reaction system in that no products could be detected after 48 h reaction time. When ball-milling cellulose (500 rpm, 4 h) was hydrolyzed in 0.08 M HCl aqueous solution (180 °C, 1 h), and the resulting hydrolysate after neutralization with NaOH was used as a substrate in the process, a lactic acid yield of 42.2% based on cellulose could be obtained (Table 2).
| Substrate | Concentration (M) | Conversion (%) | Product yield (%) | |||
|---|---|---|---|---|---|---|
| Fructose | Glucose | Glyceraldehyde | Lactic acid | |||
| a Conditions: Ba(OH)2, 0.25 M; reaction time, 48 h; nitrogen atmosphere, 1 bar total pressure. b Hydrolysate from the hydrolysis of ball-milling cellulose (500 rpm, 4 h) in 0.08 M HCl aqueous solution (180 °C, 1 h) was used as a substrate in the process. c Substrate concentration and product yields are based on glucose units in cellulose. | ||||||
| Cellobiose | 0.05 | 94.4 | 3.4 | 3.48 | 7.2 | 38.9 |
| Glucose | 0.1 | 97.4 | 3.3 | — | 5.8 | 78.3 |
| Fructose | 0.1 | 98.9 | — | 1.56 | 5.2 | 83.5 |
| Glyceraldehyde | 0.2 | 93.3 | 0.2 | 0.5 | — | 53.1 |
| Dihydroxyacetone | 0.2 | 99.7 | 0.1 | 0.4 | 2.0 | 87.0 |
| Pyruvaldehyde | 0.2 | 100 | 0 | 0 | 0 | 99.8 |
| Celluloseb,c | 0.19 | — | 1.2 | 1.5 | 11.1 | 42.2 |
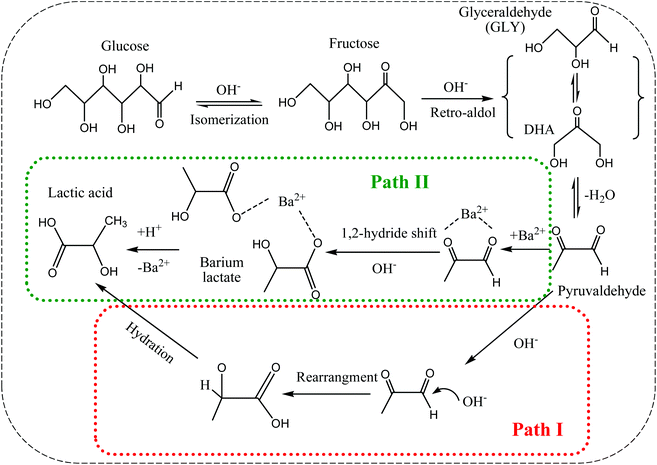
Based on the above results and the proposed reaction mechanism for the conversion of different carbohydrates such as hexoses, trioses and cellulose catalyzed by metal ions under hydrothermal conditions,13,17,18,37,38 a reaction mechanism for the conversion of glucose to lactic acid in the presence of Ba(OH)2 under a nitrogen atmosphere (anaerobic) at room temperature can be proposed (Scheme 1). Fructose is first formed by isomerization of glucose as shown in Fig. 1, followed by retro-aldol fragmentation into DHA and GLY, which both can undergo isomerization through dynamic equilibrium.18 The dehydration of DHA to pyruvaldehyde followed by a 1,2-hydride shift can be deduced as the main intermediate route for the formation of lactic acid,13 while that via dehydration of GLY plays only a minor role since the lactic acid yield from GLY was relatively low compared with that from DHA (Table 2). The catalytic activity of Ba(OH)2 should probably be attributed to its capacity to form a complex with pyruvaldehyde immediately after dehydration of DHA (Scheme 1, Path II). The complex is then transformed into lactic acid or lactate via a 1,2-hydride shift under basic conditions (Scheme 1), as this occurs in similar chemical transformations in the presence of divalent ions like Ca2+ (ref. 39), Cu2+ (ref. 37), Zn2+ (ref. 38) and Pb2+ (ref. 17). Anaerobic conditions inhibit the formation of oxidation products that can form from GLY and DHA or other intermediates that is demonstrated later. Ba(OH)2 acts both as a catalyst and reactant to form barium lactate that forces the reaction and stabilizes the lactic acid, after which the resulting barium lactate is transformed into lactic acid by addition of H2SO4 aqueous solution.16 The formed lactic acid can be separated and purified with conventional methods used in fermentation processes, such as ion exchange, liquid–liquid extraction, electrodialysis or esterification–hydrolysis.
For comparison, the results of the transformation of glucose or fructose to lactic acid or lactate in different catalytic processes are listed in Table 3. It shows that the proposed system in this work has significant advantages in terms of reaction temperature, pressure and product yield. The reaction conditions of room temperature and ambient pressure indicate that there is no specific limitation for the reactor material and many normal materials such as glass, ceramics and organic polymers can be applied for the reactor. Although the required reaction time was longer (48 h), it could be shortened to 2–6 h if the reaction temperature was elevated to approximate with that applied in the fermentation process (45–55 °C), to achieve lactic acid yields of 76–81% (Fig. S2†).The production of lactic acid for different initial glucose concentrations was investigated at 25 °C under a nitrogen atmosphere at 1 bar total pressure, and the Ba(OH)2 concentration was fixed at 0.25 M (Table 4). Glucose could be almost completely transformed into lactic acid (95.4% yield, Table 4) from a dilute glucose aqueous solution of 0.025 M in 48 h reaction time, whereas 0.05 M glucose aqueous solution afforded a lactic acid yield of 92.4%. When the initial glucose concentration was increased to 0.1 and 0.2 M, lactic acid yields of 78.3 and 54.3% were obtained, respectively, for 95% glucose conversion. However, further increase in the initial glucose concentration to 1 M resulted in a decline in the glucose conversion and negligible lactic acid formation, and almost all of the glucose was transformed into fructose (45.8% yield and 91.1% selectivity). Glucose could be quantitatively converted into lactic acid in yields above 92% as a dilute solution, but high concentrations of glucose tended to form fructose by isomerization corresponding to the effects of the alkali concentration on glucose conversion (Fig. S3–S5†).
| Substrate | Catalyst | Conditions | Solvent | Substrate concentration (M) | Substrate conversion (%) | Lactic acid/methyl lactate yield (mol%) | Ref. |
|---|---|---|---|---|---|---|---|
| Glucose | Ba(OH)2 | 25 °C, 48 h | Water | 0.025 | 99.5 | 95.4 | This work |
| Ba(OH)2 | 25 °C, 48 h | Water | 0.05 | 98 | 92.4 | This work | |
| Ba(OH)2 | 25 °C, 48 h | Water | 0.10 | 97.4 | 78.3 | This work | |
| LaCoO3 | 200 °C, 1 h | Water | 0.05 | — | 39.5 | 40 | |
| NaOH-[IMEP]Cl | 100 °C, 30 min | Water | 0.025 | 99.2 | 63 | 41 | |
| Ba(OH)2-[IMEP]Cl | 100 °C, 30 min | Water | 0.025 | 98.3 | 26 | 41 | |
| InCl3·4H2O | 200 °C, 10 h | Methanol | 0.0025 | 97 | 52 | 20 | |
| Na2SiO3 | 300 °C, 1 min | Water | 0.1 | — | 30 | 42 | |
| CuCTAB/MgO | 120 °C, 1 h, | Water | 0.0005 | >99 | 70 | 37 | |
| NaOH–NiCl2 | 300 °C, 1 min | Water | 0.097 | — | 25 | 28 | |
| Sn-MCM-41(Si/Sn = 55) | 160 °C, 20 h | Methanol | 0.125 | 100 | 43 | 43 | |
| Fructose | Ba(OH)2 | 25 °C, 48 h | Water | 0.10 | 98.7 | 83.5 | This work |
| Sn-Beta | 160 °C, 2 h | Methanol | 0.125 | 99 | 44 | 18 | |
| MoO3 + Sn-MFI | 100 °C, 48 h | Methanol | 0.044 | — | 68.2 | 44 | |
| Zr-SBA-15 | 240 °C, 6 h | Methanol | 0.056 | — | 44.1 | 45 | |
| Sn-Beta | 160 °C, 16 h | Methanol | 0.125 | 98 | 54 | 46 | |
| (C4H9)2SnO | 190 °C, 0.5 h | Water | 0.044 | — | 63 | 47 | |
| Ti-Beta | 160 °C, 20 h | Methanol | 0.125 | 99 | 36 | 18 |
| Glucose concentration (M) | Glucose conversion (%) | Product yield (%) | ||
|---|---|---|---|---|
| Fructose | Glyceraldehyde | Lactic acid | ||
| a Reaction conditions: Ba(OH)2, 0.25 M; reaction time, 48 h; temperature, 25 °C; nitrogen atmosphere, 1 bar total pressure. | ||||
| 0.025 | 99.5 | 0.17 | 0.4 | 95.4 |
| 0.05 | 98.8 | 0.55 | 1.2 | 92.5 |
| 0.1 | 97.4 | 3.33 | 5.8 | 78.3 |
| 0.2 | 94.9 | 5.77 | 3.8 | 56.6 |
| 0.5 | 72.2 | 26.8 | 1.6 | 8.60 |
| 1.0 | 50.3 | 45.8 | 0.3 | 0.88 |
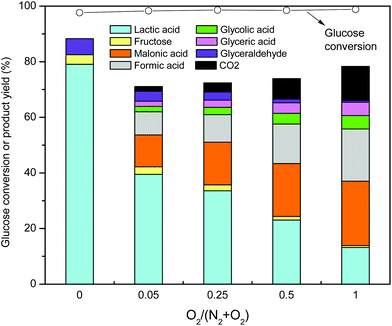
Since the formation of lactic acid from glucose in this work could be performed at room temperature and ambient pressure, the reactions were also conducted in sealed glass conical flasks, and similar results were obtained to those with Teflon-lined stainless steel reactors (Table S1†). When the reaction was carried out under an argon atmosphere at 1 bar total pressure at 25 °C, a lactic acid yield of 79.5% was obtained in 48 h, similar to the results obtained under a nitrogen atmosphere at 1 bar total pressure (Table S1†). However, when air was used as the atmosphere at 1 bar total pressure, the yield of lactic acid decreased to 61.3% in 48 h, and other organic acids such as glyceric (1.3%), glycolic (1.1%), formic (3.1%), and others appeared along with CO2 (Table S1†), demonstrating the importance of the reaction atmosphere in product formation. To understand the effect of the reaction atmosphere on the reaction system, experiments were carried out at total pressures of 20 bar that contained controlled amounts of N2 and O2 (Fig. 2). As shown in Fig. 2, glucose conversion was independent of the atmosphere composition, with about 98% glucose conversion being obtained for all cases. However, product distribution changed with the fraction of O2 in the gas atmosphere. In the absence of O2 (Fig. 2), the major product was lactic acid (79.4% yield) and minor products were fructose (3.4% yield) and GLY (5.8% yield). When 1 bar O2 partial pressure was used as the reaction atmosphere, the lactic acid yield decreased sharply to 39.5% (Fig. 2) and other organic acids (glyceric acid, glycolic acid, formic acid, malonic acid) and CO2 were formed. It became clear that the presence of O2 in the atmosphere greatly affected the solution reaction pathways most likely via O2 solubility in terms of the Henry's constant. When the O2 fraction in the atmosphere was increased (Fig. 2), the yield of lactic acid decreased, and only 13.2% could be obtained for an O2 partial pressure of 20 bar. Yields of formic acid, malonic acid, glyceric acid, glycolic acid and CO2 gradually increased with increasing O2 partial pressure (Fig. 2), which probably originates from the oxidative C–C cleavage of GLY and DHA as well as the further oxidation and decarboxylation processes. Therefore, the atmosphere plays a vital role in determining the degradation products in the reaction system, and anaerobic conditions must be strictly controlled to achieve high lactic acid yields.
Conclusions
In summary, an efficient process was developed for the quantitative conversion of glucose into lactic acid, and a lactic acid yield of 95.4% was obtained via chemocatalytic glucose conversion at room temperature and ambient pressure under anaerobic conditions. Product distribution in the Ba(OH)2 catalyzed system was greatly affected by altering the O2 partial pressure of the reaction atmosphere, such that glyceric acid, malonic acid, glycolic acid and formic acid could be formed. Thus, in the conversion of carbohydrates to lactic acid in the proposed chemocatalytic reaction system, an inert atmosphere must be strictly controlled to achieve high lactic acid yields. Moreover, the presence of O2 in solution is likely to affect product distributions in other chemocatalytic reaction systems that form acids from carbohydrate substrates. The mild conditions used for the high yield production of lactic acid makes the process attractive for potential practical applications.Acknowledgements
This work was supported by the NSFC (no. 21577073), the Natural Science Foundation of Tianjin (no. 16JCZDJC33700) and the Elite Youth program of Chinese Academy of Agricultural Sciences (to Dr Xinhua Qi).Notes and references
- A. David Martin, S. G. Wettstein and J. A. Dumesic, Chem. Soc. Rev., 2012, 41, 8075–8098 RSC.
- M. J. Climent, A. Corma and S. Iborra, Green Chem., 2014, 16, 516–547 RSC.
- H. Xiong, H. N. Pham and A. K. Datye, Green Chem., 2014, 16, 4627–4643 RSC.
- L. Qi, Y. F. Mui, S. W. Lo, M. Y. Lui, G. R. Akien and I. T. Horváth, ACS Catal., 2014, 4 Search PubMed.
- W. Deng, Q. Zhang and W. Ye, Catal. Today, 2014, 234, 31–41 CrossRef CAS.
- J. Albert, R. Wölfel, A. Bösmann and P. Wasserscheid, Energy Environ. Sci., 2012, 5, 7956–7962 CAS.
- Á. Szabolcs, M. Molnár, G. Dibó and L. T. Mika, Green Chem., 2013, 15, 439–445 RSC.
- L. Yan, N. Liu, W. Yu, H. Machida and X. Qi, Bioresour. Technol., 2014, 173, 462–466 CrossRef CAS PubMed.
- N. K. Budhavaram and Z. Fan, Bioresour. Technol., 2009, 100, 5966–5972 CrossRef CAS PubMed.
- C. Sánchez, L. Serrano, R. Llano-Ponte and J. Labidi, Chem. Eng. J., 2014, 250, 326–330 CrossRef.
- R. Datta and M. Henry, J. Chem. Technol. Biotechnol., 2006, 81, 1119–1129 CrossRef CAS.
- K. Press, I. Goldberg and M. Kol, Angew. Chem., Int. Ed., 2015, 54, 14858–14861 CrossRef CAS PubMed.
- M. A. PaIvi, I. L. Simakova, S. Tapio and M. Dmitry Yu, Chem. Rev., 2014, 114, 1909–1971 CrossRef PubMed.
- K. Okano, T. Tanaka, C. Ogino, H. Fukuda and A. Kondo, Appl. Microbiol. Biotechnol., 2010, 85, 413–423 CrossRef CAS PubMed.
- M. Dusselier, P. V. Wouwe, A. Dewaele, E. Makshina and B. F. Sels, Energy Environ. Sci., 2013, 6, 1415–1442 CAS.
- D. Esposito, ChemSusChem, 2013, 6, 989–992 CrossRef CAS PubMed.
- Y. Wang, W. Deng, B. Wang, Q. Zhang, X. Wan, Z. Tang, Y. Wang, C. Zhu, Z. Cao and G. Wang, Nat. Commun., 2013, 4, 2141–2141 Search PubMed.
- M. S. Holm, S. Saravanamurugan and E. Taarning, Science, 2010, 328, 602–605 CrossRef CAS PubMed.
- B. Murillo, B. Zornoza, O. de la Iglesia, C. Tellez and J. Coronas, J. Catal., 2016, 334, 60–67 CrossRef CAS.
- K. Nemoto, Y. Hirano, K.-i. Hirata, T. Takahashi, H. Tsuneki, K.-i. Tominaga and K. Sato, Appl. Catal., B, 2016, 183, 8–17 CrossRef CAS.
- M. Morales, P. Y. Dapsens, I. Giovinazzo, J. Witte, C. Mondelli, S. Papadokonstantakis, K. Hungerbuhler and J. Perez-Ramirez, Energy Environ. Sci., 2015, 8, 558–567 CAS.
- R. M. West, M. S. Holm, S. Saravanamurugan, J. Xiong, Z. Beversdorf, E. Taarning and C. H. Christensen, J. Catal., 2010, 269, 122–130 CrossRef CAS.
- C. B. Rasrendra, B. A. Fachri, S. Adisasmito and P. H. J. Heeres, ChemSusChem, 2011, 4, 768–777 CrossRef CAS PubMed.
- P. Y. Dapsens, C. Mondelli and J. Pérez-Ramírez, ChemSusChem, 2013, 6, 831–839 CrossRef CAS PubMed.
- F. de Clippel, M. Dusselier, R. Van Rompaey, P. Vanelderen, J. Dijkmans, E. Makshina, L. Giebeler, S. Oswald, G. V. Baron, J. F. M. Denayer, P. P. Pescarmona, P. A. Jacobs and B. F. Sels, J. Am. Chem. Soc., 2012, 134, 10089–10101 CrossRef CAS PubMed.
- S. Lux and M. Siebenhofer, Catal. Sci. Technol., 2013, 3, 1380–1385 CAS.
- D. Tongsakul, S. Nishimura and K. Ebitani, ACS Catal., 2013, 3, 2199–2207 CrossRef CAS.
- Z. Huo, Y. Fang, D. Ren, S. Zhang, G. Yao, X. Zeng and F. Jin, ACS Sustainable Chem. Eng., 2014, 2, 2765–2771 CrossRef CAS.
- C. B. Rasrendra, I. G. B. N. Makertihartha, S. Adisasmito and H. J. Heeres, Top. Catal., 2010, 53, 1241–1247 CrossRef CAS.
- F. Chambon, F. Rataboul, C. Pinel, A. Cabiac, E. Guillon and N. Essayem, Appl. Catal., B, 2011, 105, 171–181 CrossRef CAS.
- J. Duo, Z. Zhang, G. Yao, Z. Huo and F. Jin, Catal. Today, 2016, 263, 112–116 CrossRef CAS.
- C. Sanchez, I. Eguees, A. Garcia, R. Llano-Ponte and J. Labidi, Chem. Eng. J., 2012, 181, 655–660 CrossRef.
- X. Yan, F. Jin, K. Tohji, A. Kishita and H. Enomoto, AIChE J., 2010, 56, 2727–2733 CrossRef CAS.
- F. M. Jin and H. Enomoto, Energy Environ. Sci., 2011, 4, 382–397 CAS.
- T. Meilin, Y. Xiaohu, I. Delidovich, P. Regina, S. Junyou and W. Xiaohong, ChemSusChem, 2015, 8, 4195–4201 CrossRef PubMed.
- Z. Tang, W. Deng, Y. Wang, E. Zhu, X. Wan, Q. Zhang and Y. Wang, ChemSusChem, 2014, 7, 1557–1567 CrossRef CAS PubMed.
- H. Choudhary, S. Nishimura and K. Ebitani, Appl. Catal., B, 2015, 162, 1–10 CrossRef CAS.
- M. Bicker, S. Endres, L. Ott and H. Vogel, J. Mol. Catal. A: Chem., 2005, 239, 151–157 CrossRef CAS.
- W. Jeon, C. Ban, G. Park, H. C. Woo and D. H. Kim, Catal. Sci. Technol., 2015, 6, 1146–1156 Search PubMed.
- X. Yang, L. Yang, W. Fan and H. Lin, Catal. Today, 2016, 269, 56–64 CrossRef CAS.
- X. C. Wang, Y. L. Song, C. P. Huang, F. B. Liang and B. H. Chen, Green Chem., 2014, 16, 4234–4240 RSC.
- D. Jia, Z. Zhang, G. Yao, Z. Huo and F. Jin, Catal. Today, 2016, 263, 112–116 CrossRef.
- B. Murillo, A. Sánchez, V. Sebastián, C. Casado-Coterillo, D. L. I. Oscar, C. Téllez and J. Coronas, J. Chem. Technol. Biotechnol., 2014, 89, 1344–1350 CrossRef CAS.
- M. Orazov and M. E. Davis, Proc. Natl. Acad. Sci. U. S. A., 2015, 112 Search PubMed.
- L. Yang, X. Yang, E. Tian, V. Vattipalli, W. Fan and H. Lin, J. Catal., 2016, 333, 207–216 CrossRef CAS.
- M. S. Holm, Y. J. Pagán-Torres, S. Saravanamurugan, A. Riisager, J. A. Dumesic and E. Taarning, Green Chem., 2012, 14, 702–706 RSC.
- J. B. D. Santos and M. R. Meneghetti, RSC Adv., 2015, 5, 90952–90959 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6gc02443b |
| This journal is © The Royal Society of Chemistry 2017 |