Structure characterization of a novel polysaccharide from Hericium erinaceus fruiting bodies and its immunomodulatory activities
Fangfang
Wu
 a,
Chunhui
Zhou
b,
Dandan
Zhou
b,
Shiyi
Ou
c,
Xiaoai
Zhang
d and
Huihua
Huang
*a
a,
Chunhui
Zhou
b,
Dandan
Zhou
b,
Shiyi
Ou
c,
Xiaoai
Zhang
d and
Huihua
Huang
*a
aSchool of Food Science and Engineering, South China University of Technology, Guangzhou 510641, China. E-mail: fehhuang@scut.edu.cn; Fax: +86-20-87112851; Tel: +86-20-87112851
bGuangdong Apollo Group Co., Ltd, Guangzhou 510665, China
cDepartment of Food Science and Engineering, Jinan University, Guangzhou 510632, China
dAgrobiological Gene Research Center, Guangdong Academy of Agricultural Sciences, Guangzhou 510640, China
First published on 9th November 2017
Abstract
A novel polysaccharide fraction (HEP-S) was extracted and isolated from the fruiting bodies of Hericium erinaceus. Structural characterization revealed that HEP-S had an average molecular weight of 1.83 × 104 Da and consisted of rhamnose, fucose, mannose, glucose and galactose at a molar ratio of 1.47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.93
0.93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.36
1.36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.68
8.68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.08. Periodate oxidation–Smith degradation and NMR analysis showed that the main linkage types of HEP-S were composed of (1→)-α-D-Glc, (1→3,4)-α-D-Glc, (1→6)-α-D-Gal, (1→3,4)-β-D-Man, (1→3,6)-α-Rha and (1→2)-β-L-Fuc. The immunomodulatory assay indicated that HEP-S could significantly enhance the pinocytic and phagocytic capacity and promote the secretion of nitric oxide and pro-inflammatory cytokines by activating the corresponding mRNA and protein expression in RAW 264.7 cells involving a toll-like receptor 2 membrane receptor. Besides, HEP-S was also found to improve the adaptive immune function by enhancing T and B lymphocyte proliferation and increasing the interleukin-2, interleukin-4 and interferon-γ secretion in spleen lymphocytes. These results suggested that HEP-S could be used as a potential immunoregulatory agent in functional foods.
4.08. Periodate oxidation–Smith degradation and NMR analysis showed that the main linkage types of HEP-S were composed of (1→)-α-D-Glc, (1→3,4)-α-D-Glc, (1→6)-α-D-Gal, (1→3,4)-β-D-Man, (1→3,6)-α-Rha and (1→2)-β-L-Fuc. The immunomodulatory assay indicated that HEP-S could significantly enhance the pinocytic and phagocytic capacity and promote the secretion of nitric oxide and pro-inflammatory cytokines by activating the corresponding mRNA and protein expression in RAW 264.7 cells involving a toll-like receptor 2 membrane receptor. Besides, HEP-S was also found to improve the adaptive immune function by enhancing T and B lymphocyte proliferation and increasing the interleukin-2, interleukin-4 and interferon-γ secretion in spleen lymphocytes. These results suggested that HEP-S could be used as a potential immunoregulatory agent in functional foods.
1. Introduction
Hericium erinaceus (H. erinaceus) belongs to the Aphyllophorales order and Hydnaceae family, and is a traditional food source and medicinal fungus in Asian countries and even the USA and Europe.1 Recently, H. erinaceus has attracted great attention in the fields of functional food and biomedical science due to its various beneficial effects. Usually, polysaccharides are well documented to be a major active ingredient of H. erinaceus, which possess various pharmacological activities such as antitumor activity,2,3 anti-proliferation property,4 antioxidant property,4–6 hepatoprotective effect6 and anti-chronic atrophic gastritis activity.7 To date, a couple of polysaccharides with various bioactivities have been purified from the fruiting bodies and mycelia of H. erinaceus, but little information is available about their chemical structure and immunomodulatory activities.For a living organism, immunity is defined as the ability to recognize and destroy external harmful substances,8 which plays a crucial role in our health. Classically, immune responses are divided into innate and adaptive immunity with different roles and functions. It is well known that the innate immune response is the first line of host protection in the defense against invading and infecting microbial pathogens and is mediated primarily by macrophages, granulocytes, monocytes and dendritic cells.9 The adaptive immune response, on the other hand, is responsible for the elimination of pathogens and for the generation of immunological memory.10,11 In most cases, macrophages and lymphocytes are usually used as the ideal cell models to evaluate the immunity of bioactive components.
Macrophages are distributed throughout phylogenetically conserved cells in all the multicellular organisms. They can neutralize foreign substances, cancer cells and infectious microbes through phagocytosis, chemotaxis, and surveillance and by releasing proinflammatory cytokines and cytotoxic components, such as nitric oxide (NO), tumor necrosis factor-alpha (TNF-α), reactive oxygen species (ROS), interleukin (IL)-6, etc.12–15 Meanwhile, as the major cellular components of the immune response, lymphocytes can constitute memory cells for a specific antigen, which can be activated after a process known as antigen presentation by antigen-presenting cells.16 Indeed, cell proliferation activity is one of the most important functions of lymphocytes, and the cellular proliferation induced by concanavalin-A (ConA) and lipopolysaccharide (LPS) is commonly used to detect T and B lymphocyte immunity, respectively.17 In particular, multiple cytokines, e.g. IL-2, IL-4 and IFN-γ, can be used to test the effect of modulation on lymphocytes.
In the present study, a novel polysaccharide fraction (HEP-S) was extracted, isolated and purified from the fruiting bodies of H. erinaceus and its primary structural characteristics including molecular weight, linkage information and monosaccharide composition were analyzed. The immunomodulatory activity of HEP-S was then examined using the murine macrophage RAW 264.7 cells and spleen lymphocyte model system. The effects on the pinocytic and phagocytic capacity, production of ROS, TNF-α, NO and IL-6, relevant messenger ribonucleic acid (mRNA) expression and protein expression of the cells were detected. Furthermore, the membrane receptors of HEP-S on the RAW 264.7 cell line were also explored. Finally, we also determined the secretion level of cytokines, including IL-2, IL-4 and IFN-γ, in spleen lymphocytes.
2. Materials and methods
2.1. Materials and chemicals
H. erinaceus was obtained from Pingnan H. erinaceus plantation (Pingnan country, Fujian Province, China). The RAW 264.7 cell line was purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). DEAE-Sepharose fast flow and Sephadex G-100 were purchased from GE Healthcare Life Science (Piscataway, NJ). Standard monosaccharides (including glucuronic acid, rhamnose, arabinose, fucose, xylose, mannose, glucose, galactose and inositol) were obtained from Shanghai Yuanye Bio-Technology Co. Ltd (Shanghai, China). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS, pH 7.4), streptomycin and penicillin were acquired from Gibco Life Technologies (Waltham, MA, USA). 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) was obtained from Shanghai Bestbio Biotechnology Inc. (Shanghai, China). LPS, laminarin, neutral red, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), ConA, glycerol, erythritol, ethylene glycol, various dextran standards and bovine serum albumin (BSA) were obtained from Sigma-Aldrich Co. (St Louis, USA). Trizol was acquired from Invitrogen Life Technologies (California, USA). First Strand cDNA Synthesis Kit and FastStart Universal SYBR Green Master (ROX) were obtained from F. Hoffmann-La Roche Ltd (Basel, Switzerland). Mouse IL-2, IL-4, IL-6, TNF-α, and IFN-γ enzyme-linked immunosorbent assay (ELISA) kits were purchased from Neobioscience Technology Co., Ltd (Shenzhen, China). NO detecting kit was purchased from Nanjing Jiancheng Institute of Biotechnology (Nanjing, China). ROS assay kit was obtained from Beijing Solarbio Science and Technology Co., Ltd (Beijing, China). Anti-mannose receptor antibody (anti-MR), anti-scavenger receptor I antibody (anti-SR), anti-toll-like 2 antibody (anti-TLR2), anti-toll-like 4 receptor antibody (anti-TLR4), anti-beta glucan receptor antibody (anti-GR), anti-complement receptor 3 antibody (anti-CR3) and anti-TNF-α antibody were obtained from Abcam, Inc. (Cambridge, MA, USA). Antibodies against β-actin, iNOS and IL-6 were provided by Cell Signaling Technology (Beverly, MA, USA). All other chemicals and solvents used in the experiment were of analytical reagent grade.2.2. Extraction and purification of polysaccharide from the fruiting bodies of H. erinaceus
The dried fruiting bodies of H. erinaceus were pulverized and passed through a 60 mesh sieve. The powder was extracted with distilled water at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/v) at 85 °C twice (2.5 h each time). The aqueous extracts were centrifuged at 3000g for 20 min to remove the residue. The supernatant was collected and concentrated to one-fifth of the initial volume at reduced pressure and then was precipitated by ethanol addition to a final concentration of 75% (v/v) at 4 °C overnight. The precipitate was separated and collected by centrifugation at 5000g for 20 min. After that, the protein in the precipitate was removed by using the Sevag method. Finally, the precipitate was dialyzed in distilled water for 48 h at 4 °C and then freeze-dried to obtain a crude polysaccharide.
20 (w/v) at 85 °C twice (2.5 h each time). The aqueous extracts were centrifuged at 3000g for 20 min to remove the residue. The supernatant was collected and concentrated to one-fifth of the initial volume at reduced pressure and then was precipitated by ethanol addition to a final concentration of 75% (v/v) at 4 °C overnight. The precipitate was separated and collected by centrifugation at 5000g for 20 min. After that, the protein in the precipitate was removed by using the Sevag method. Finally, the precipitate was dialyzed in distilled water for 48 h at 4 °C and then freeze-dried to obtain a crude polysaccharide.
The crude polysaccharide (150 mg) was dissolved in 10 mL of ultrapure water and loaded onto a pre-equilibrated DEAE-Sepharose fast flow anion exchange column (3.5 cm × 20 cm). The column was eluted with distilled water and 0.05, 0.1, 0.2, 0.3 and 0.5 M NaCl solutions at 1.0 mL min−1. HEP-S (0.05 M NaCl salt elution) was dialyzed, freeze-dried and further purified by Sephadex G-100 column chromatography (1.6 cm × 60 cm). The sample was eluted with ultrapure water at a rate of 0.5 mL min−1. The elution was detected using the phenol–sulfuric acid method. Then the solution was dialyzed in distilled water for 48 h at 4 °C. After freeze-drying, HEP-S was available for the subsequent experiments.
2.3. Structure characterization of HEP-S
The meta-hydroxydiphenyl colorimetric method was used to detect the uronic acid content of polysaccharides using D-glucuronic acid as a standard.18
The acetate derivative was analyzed using an Agilent 7890A gas chromatography system (Agilent Technologies, USA) with a HP-5 capillary column (30 m × 0.32 mm × 0.25 μm). The initial temperature was set at 150 °C, and was linearly increased to 200 °C at 2.5 °C min−1, and then maintained for 12 min. The injector and detector temperature was set at 250 °C. A set of monosaccharides (glucuronic acid, rhamnose, arabinose, fucose, xylose, mannose, glucose, and galactose) were used as standards and 1.0 mg mL−1 of inositol was used as the internal standard.
2.4. Effects of HEP-S on lymphocyte immunomodulatory activities
2.5. Effects of HEP-S on macrophage immunomodulatory activities
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol = 1
ethanol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The cell culture plate was statically placed for 2 h at room temperature. The absorbance at 540 nm was measured using a microplate reader (Biotek, USA).
1). The cell culture plate was statically placed for 2 h at room temperature. The absorbance at 540 nm was measured using a microplate reader (Biotek, USA).
2.6. Statistical analysis
The data were expressed as means ± standard deviation (S ± D) for three replicates. One way ANOVA was used to analyze the significant differences between the groups by SPSS 20.0. p < 0.05 was accepted as the level of significance.3. Results and discussion
3.1. Extraction, purification and structural characterization of HEP-S
Crude polysaccharides were isolated from the dried fruiting bodies of H. erinaceus with a yield of 2.56 ± 0.14% (w/w). After purification with a DEAE-Sepharose fast flow column (Fig. 1a), two major fractional peaks, eluted with distilled water (HEP-W) and 0.05 M NaCl (HEP-S), were obtained. In this study, we mainly focused on the HEP-S fraction, and the other HEP-W fraction will be studied in our future work. Fig. 1(b) shows that HEP-S appeared as a single and symmetrical peak after being further purified by Sephadex G-100 column chromatography. After dialysis and lyophilization, the total sugar content and uronic acid content of HEP-S were detected to be 92.53% ± 0.78% (w/w) and 0.56% ± 0.08%, respectively.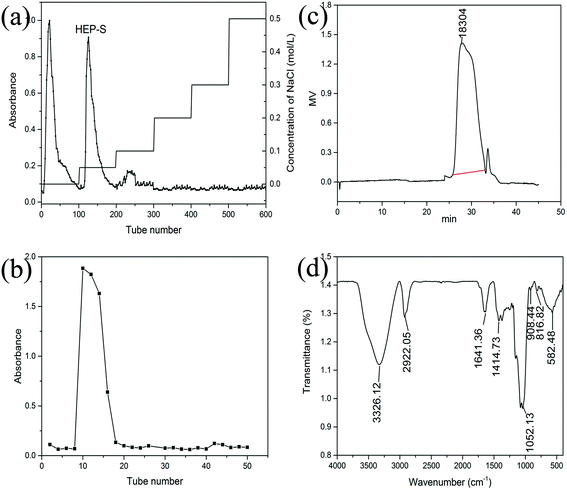 | ||
| Fig. 1 Chromatograms of HEP-S from H. erinaceus from (a) DEAE-Sepharose fast flow chromatography, (b) Sephadex G-100 chromatography and (c) HPGPC, and (d) FT-IR spectrum. | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching and pyranose ring, respectively. A characteristic absorption at 908.44 cm−1 was also observed, indicating the β-pyranoside linkage. A characteristic peak at 582.48 cm−1 indicated the existence of α-configurations.
C stretching and pyranose ring, respectively. A characteristic absorption at 908.44 cm−1 was also observed, indicating the β-pyranoside linkage. A characteristic peak at 582.48 cm−1 indicated the existence of α-configurations.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.93
0.93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.36
1.36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.68
8.68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.08, indicating that HEP-S was a kind of a heteropolysaccharide with glucose occupying the biggest proportion. These results were different from that of Zhang et al., who found that the polysaccharide from H. erinaceus consisted of fucose, galactose and glucose at a molar ratio of 1
4.08, indicating that HEP-S was a kind of a heteropolysaccharide with glucose occupying the biggest proportion. These results were different from that of Zhang et al., who found that the polysaccharide from H. erinaceus consisted of fucose, galactose and glucose at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25
1.25
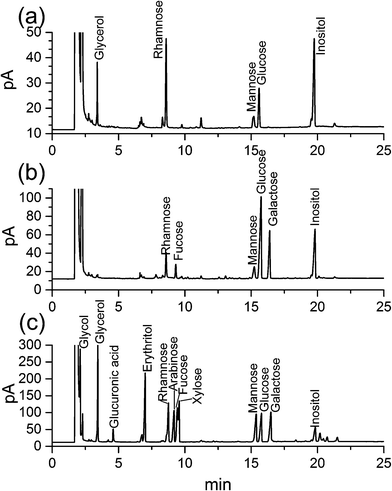
After further Smith degradation of the oxidized HEP-S, glycerol, rhamnose, mannose, glucose were detected by GC at a molar ratio of 2.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.43
3.43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.96
0.96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.99 (Fig. 2a). The presence of glycerol indicated the existence of (1→), (1→6) and (1→2)-linked glycosidic bonds. In addition, the presence of monosaccharides revealed the appearance of (1→3), (1→3,4) and (1→3,6)-linked glycosidic bonds.26,27
1.99 (Fig. 2a). The presence of glycerol indicated the existence of (1→), (1→6) and (1→2)-linked glycosidic bonds. In addition, the presence of monosaccharides revealed the appearance of (1→3), (1→3,4) and (1→3,6)-linked glycosidic bonds.26,27
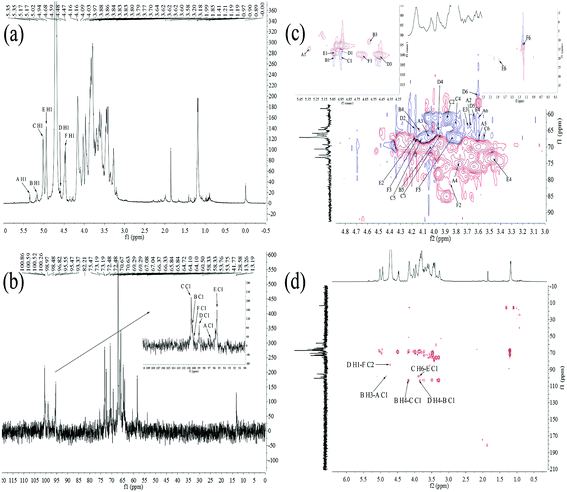
| Residue | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 |
|---|---|---|---|---|---|---|
| α-D-Glc-(1→A | 5.35/98.17 | 3.58/65.80 | 3.97/67.08 | 3.77/75.47 | 3.84/67.08 | 3.64/60.63 |
| →3,4)-α-D-Glc-(1→B | 5.17/102.67 | —/— | 4.81/98.48 | 4.16/64.10 | 4.09/69.29 | —/— |
| →6)-α-D-Gal-(1→C | 5.02/102.66 | 3.86/58.33 | 4.09/66.35 | 3.84/64.72 | 3.97/67.00 | 3.83/67.04 |
| →3,4)-β-D-Man-(1→D | 4.68/98.97 | 4.14/64.72 | 4.47/100.52 | 3.84/66.37 | 3.64/60.50 | 3.70/53.75 |
| →3,6)-α-Rha-(1→E | 4.94/98.05 | 4.15/66.33 | 3.80/65.84 | 3.20/73.19 | —/— | 1.85/28.58 |
| →2)-β-L-Fuc-(1→F | 4.59/100.26 | 3.88/82.21 | 4.17/66.33 | 3.62/60.50 | 3.86/65.80 | 1.21/13.26 |
In the 1H–13C HMBC spectrum of HEP-S (Fig. 3(d)), cross signal 3.83/98.05 ppm was attributed to C-1 (δ 98.05 ppm) for residue E and H-6 (δ 3.83 ppm) for residue C, indicating that C-1 of residue E was linked to O-6 of residue C.37 Meanwhile, the signals of C-1 at 102.66 ppm of C were related to the H-4 signals at 4.16 ppm of B, indicating the presence of →6)-α-D-Galp-(1→4)-α-D-Glcp-(1→. The cross signal of C-1 at 102.67 ppm of B and H-4 at 3.84 ppm of D showed the presence of →3,4)-α-D-Glcp-(1→4)-β-D-Manp-(1→. The C-2 signal at 82.21 ppm of F was correlated with the H-1 signal at 4.68 ppm of D. Thus, it was possible to conclude that a repeating unit of HEP-S contained a backbone structure of E-(1→6)-C-(1→4)-B-(1→4)-D-(1→2)-F. Meanwhile, the presence of the cross signal 4.81/98.17 ppm indicated that C-1 (δ 98.17 ppm) of residue A was linked to O-3 (δ 4.81 ppm) of residue B. Furthermore, the chemical shifts at 170–180 ppm were also detected in the HMBC spectrum (Fig. 3(d)), further indicating the presence of uronic acid.38
Based on the above results, HEP-S could be identified to be a branched polysaccharide and the main backbone of HEP-S was identified as →3,6)-α-Rhap-(1→6)-α-D-Galp-(1→4)-α-D-Glcp-(1→4)-β-D-Manp-(1→2)-β-L-Fucp-(1→ with a branch that was composed of α-D-Glcp-residues. The structure was markedly different from other reported polysaccharides derived from the fruiting bodies of H. erinaceus. For example, a heteropolysaccharide (HEPF1)25 was confirmed to contain a (1→6)-linked α-D-galactopyranosyl backbone with branches that were composed of fucose attached to O-2, it also contained 6-O-substituted-β-D-oligoglucosyl units and a minor terminal 3-O-methyl rhamnose residue. In fact, different structural characteristics might contribute to different bioactivities of H. erinaceus polysaccharides.
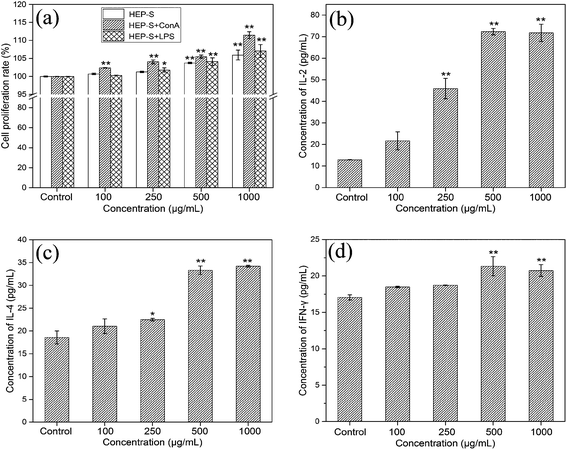
3.2. Immunomodulatory activities of HEP-S on spleen lymphocytes
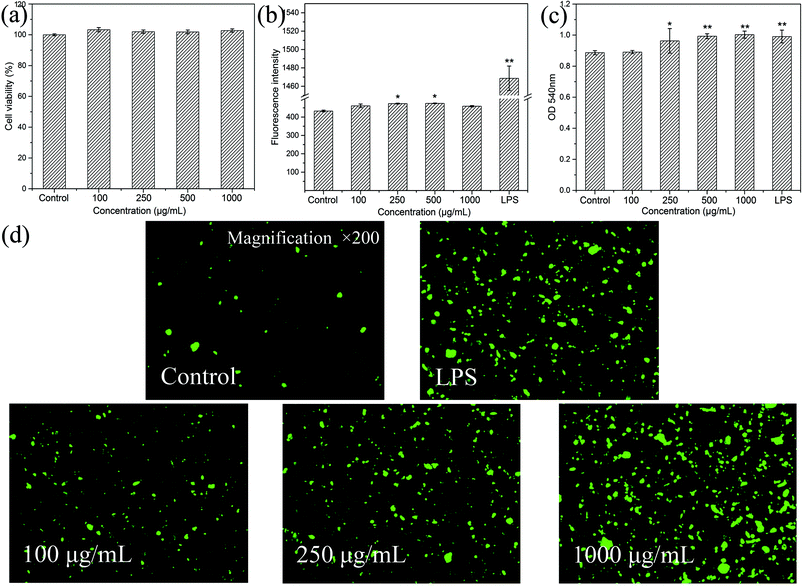
3.3. Immunomodulatory activities of HEP-S on RAW 264.7 cells
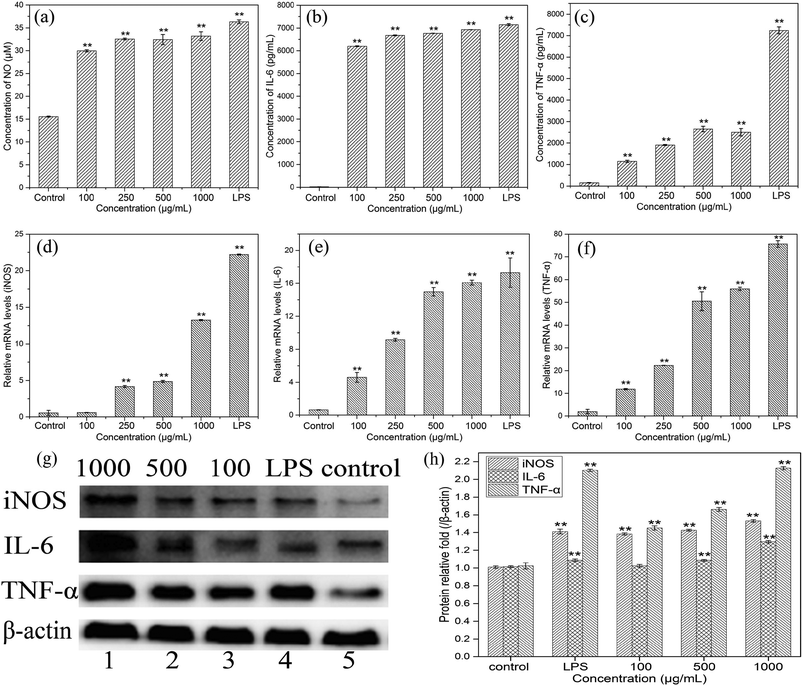
To evaluate the effects of HEP-S on the mRNA expression of inducible nitric oxide synthase (iNOS) and cytokines in RAW 264.7 cells, the gene transcription of iNOS, IL-6 and TNF-α was further examined by RT-PCR. As shown in Fig. 6(d–f), compared to the control group, the cells treated with HEP-S or LPS also showed a significant increase in the mRNA levels of iNOS, IL-6 and TNF-α, which was consistent with the NO, IL-6 and TNF-α secretion levels.
In order to further elucidate the effects of HEP-S on the protein expression of iNOS, IL-6 and TNF-α in RAW 264.7 cells, western blot analysis was used in this study. As shown in Fig. 6(g and h), compared to the negative control group (lane 5), the cells treated with HEP-S or LPS showed significant expression in protein levels (p < 0.05). The results suggested that the increase in the NO, IL-6 and TNF-α secretion levels in the activated murine macrophages might be due to the enhancement of the corresponding mRNA and protein expression.
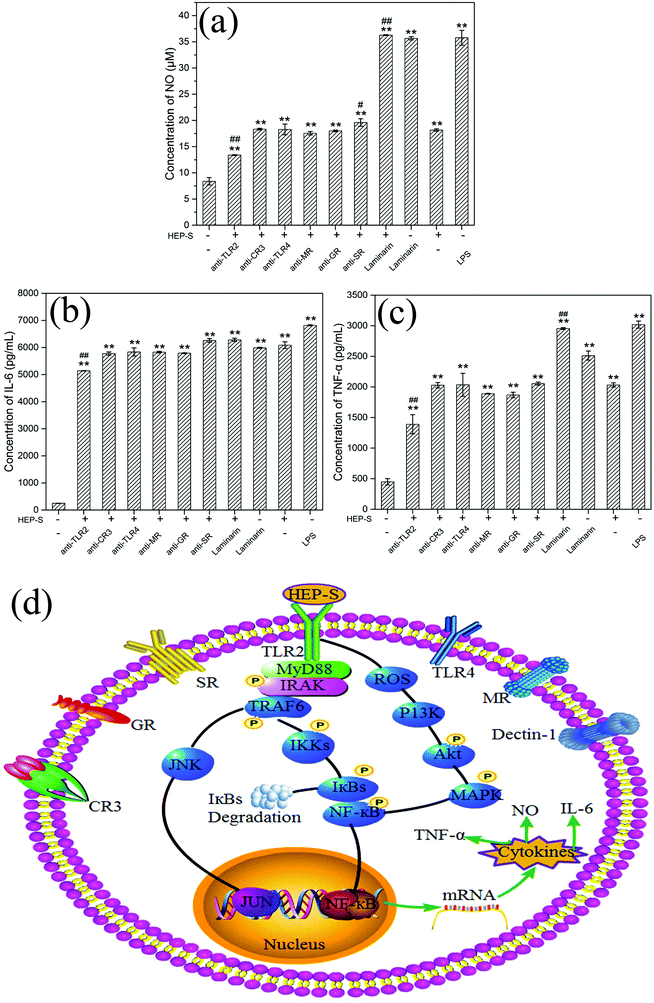
To determine whether TLR2, CR3, TLR4, MR, GR, SR and dectin-1 play a role in HEP-S-mediated stimulation in RAW 264.7 cells, these antibodies were used in this study. The results are shown in Fig. 7(a–c). It was found that after the treatment of anti-TLR2 and HEP-S, the cells showed a significant decrease in the levels of NO, IL-6, and TNF-α as compared to the group only treated with HEP-S (p < 0.01). However, no significant decrease in the levels of NO, IL-6, and TNF-α was observed in the groups treated with anti-CR3, anti-TLR4, anti-MR, anti-GR, anti-SR and laminarin (dectin-1 inhibitor). Meanwhile, it was noted that the levels of NO, IL-6, and TNF-α in the group treated with anti-TLR2 were still maintained significantly higher than those in the control group (p < 0.01), suggesting that TLR2 was one of the pattern recognition receptors for HEP-S in macrophages to induce transcriptional activation and expression of cytokines, while other immune receptors also exist. Indeed, these seven receptors were a small part of RPPs on macrophages, so the receptors of HEP-S need to be further investigated in our future work.
It has been reported that TLR2 can interact with a series of microbial ligands and macromolecules, which subsequently allows macrophages to exhibit self-polarization and differentiation to further promote the maturation and development of immunity.45,46 A number of studies have demonstrated the TLR2-dependent activation of macrophages by mushroom polysaccharides. For instance, Xu et al. reported that a new heteropolysaccharide fraction from Lentinula edodes (L2) stimulated IL-6 levels via the TLR2 activation of the signaling pathway in RAW 264.7 cells.43 Gantner et al. also indicated that lentian exhibited its immunomodulating effects via binding with TLR2.47
Based on the previous study,48 TLR2 has been proven to be involved in the activation of IL-1R-associated kinase (IRAK) via an adaptor myeloid differentiation protein 88 (MyD88) with the subsequent activation of TNF receptor associated factor 6 (TRAF-6), Jun N-terminal kinase (JNK) and nuclear factor (NF)-κB. Meanwhile, increasing evidence indicated that ROS could not only play roles in host defense but also act as a cellular secondary messenger in many biological functions, such as transcription factor activation, protein phosphorylation and cytokine production.49–51 Yu et al. indicated that ROS was involved in the activation of phosphoinositide-3-kinase (PI3K), activation of the protein kinase B (Akt) and mitogen-activated protein kinase (MAPK), and NF-κB signal transduction pathways (PI3K/Akt/MAPKs/NF-κB pathway).52 The activation of these transcription pathways leads to gene transcription and the production of iNOS and cytokines. Based on these discussions, we can infer that the possible mechanism of HEP-S-induced macrophage immunomodulatory activity is mainly via two signaling pathways (Fig. 7).
4. Conclusions
In this study, a new fraction of a heteropolysaccharide (HEP-S) with an average molecular weight of 1.83 × 104 Da was isolated from the fruiting bodies of Hericium erinaceus and its primary chemical structure was characterized. The bioactivity tests showed that HEP-S possessed a significant immunomodulatory activity by enhancing the pinocytic and phagocytic capacity and increasing NO, IL-6, and TNF-α secretion by activating the corresponding mRNA expression and protein expression in RAW 264.7 cells, and TLR2 was confirmed to be one of the major membrane receptors of HEP-S. Besides, HEP-S was also found to improve the adaptive immune function by enhancing T and B lymphocyte proliferation and increasing the IL-2, IL-4 and IFN-γ secretion in spleen lymphocytes. These results suggested that HEP-S can function as an immunostimulator to stimulate both the innate and adaptive immune responses and might be a potential candidate for application in immunological diseases and functional foods.Funding
The authors are grateful for the financial support from the Guangdong Provincial Department of Science and Technology, China (Grant No. 2013B090600008).Conflicts of interest
The authors declare no conflicts of interest in this research manuscript.References
- J. S. Lee, J. Y. Cho and E. K. Hong, Study on macrophage activation and structural characteristics of purified polysaccharides from the liquid culture broth of Hericium erinaceus, Carbohydr. Polym., 2009, 78, 162–168 CrossRef CAS.
- J. S. Lee and E. K. Hong, Hericium erinaceus, enhances doxorubicin-induced apoptosis in human hepatocellular carcinoma cells, Cancer Lett., 2010, 297, 144–154 CrossRef CAS PubMed.
- S. P. Kim, M. Y. Kang, J. H. Kim, S. H. Nam and M. Friedman, Composition and mechanism of antitumor effects of Hericium erinaceus, mushroom extracts in tumor-bearing mice, J. Agric. Food Chem., 2011, 59, 9861–9869 CrossRef CAS PubMed.
- X. Y. Li, Z. Y. Wang, L. Wang, E. Walid and H. Zhang, In vitro antioxidant and anti-proliferation activities of polysaccharides from various extracts of different mushrooms, Int. J. Mol. Sci., 2012, 13, 5801–5817 CrossRef CAS PubMed.
- Z. H. Han, J. M. Ye and G. F. Wang, Evaluation of in vivo antioxidant activity of Hericium erinaceus polysaccharides, Int. J. Biol. Macromol., 2013, 52, 66–71 CrossRef CAS PubMed.
- Z. Zhang, G. Lv, H. Pan, A. Pandey, W. He and L. Fan, Antioxidant and hepatoprotective potential of endo-polysaccharides from Hericium erinaceus grown on tofu whey, Int. J. Biol. Macromol., 2012, 51, 1140–1146 CrossRef CAS PubMed.
- M. Wang, Y. Gao, D. Xu and Q. Gao, A polysaccharide from cultured mycelium of Hericium erinaceus, and its anti-chronic atrophic gastritis activity, Int. J. Biol. Macromol., 2015, 81, 656–661 CrossRef CAS PubMed.
- J. Trowsdale and P. Parham, Mini-review: Defense strategies and immunity-related genes, Eur. J. Immunol., 2004, 34, 7–17 CrossRef CAS PubMed.
- S. Z. Xie, R. Hao, X. Q. Zha, L. H. Pan, J. Liu and J. P. Luo, Polysaccharide of Dendrobium huoshanense activates macrophages via toll-like receptor 4-mediated signaling pathways, Carbohydr. Polym., 2016, 146, 292–300 CrossRef CAS PubMed.
- T. H. Mogensen, Pathogen recognition and inflammatory signaling in innate immune defenses, Clin. Microbiol. Rev., 2009, 22, 240–273 CrossRef CAS PubMed.
- H. Rabb, The T cell as a bridge between innate and adaptive immune systems: implications for the kidney, Kidney Int., 2002, 61, 1935–1946 CrossRef CAS PubMed.
- M. M. Zhang, G. Wang, F. R. Lai and H. Wu, Structural characterization and immunomodulatory activity of a novel polysaccharide from Lepidium meyenii, J. Agric. Food Chem., 2016, 64, 1921–1931 CrossRef CAS PubMed.
- W. Z. Liao, Z. Luo, D. Liu, Z. X. Ning, J. G. Yang and J. Y. Ren, Structure characterization of a novel polysaccharide from Dictyophora indusiata and its macrophage immunomodulatory activities, J. Agric. Food Chem., 2015, 63, 535–544 CrossRef CAS PubMed.
- F. F. Wu, C. H. Zhou, D. D. Zhou, S. Y. Ou and H. H. Huang, Structural characterization of a novel polysaccharide fraction from Hericium erinaceus and its signaling pathways involved in macrophage immunomodulatory activity, J. Funct. Foods, 2017, 37, 574–585 CrossRef CAS.
- K. L. Cheong, L. Z. Meng, X. Q. Chen, L. Y. Wang, D. T. Wu, J. Zhao and S. P. Li, Structural elucidation, chain conformation and immuno-modulatory activity of glucogalactomannan from cultured Cordyceps sinensis, fungus UM01, J. Funct. Foods, 2016, 25, 174–185 CrossRef CAS.
- Q. Yu, S. P. Nie, J. Q. Wang, D. F. Huang, W. J. Li and M. Y. Xie, Signaling pathway involved in the immunomodulatory effect of Ganoderma atrum, polysaccharide in spleen lymphocytes, J. Agric. Food Chem., 2015, 63, 2734–2740 CrossRef CAS PubMed.
- J. X. Wang, X. Tong, P. B. Li, H. Cao and W. W. Su, Immuno-enhancement effects of Shenqi Fuzheng Injection on cyclophosphamide-induced immunosuppression in Balb/c mice, J. Ethnopharmacol., 2012, 139, 788–795 CrossRef PubMed.
- T. M. Filisetti-Cozzi and N. C. Carpita, Measurement of uronic acids without interference from neutral sugars, Anal. Biochem., 1991, 197, 157 CrossRef CAS PubMed.
- Z. Lin, W. Liao and J. Ren, Physicochemical characterization of a polysaccharide fraction from Platycladus orientalis, (L.) franco and its macrophage immunomodulatory and anti-hepatitis B virus activities, J. Agric. Food Chem., 2016, 64, 5813–5823 CrossRef CAS PubMed.
- R. Dong, X. Wang, H. Wang, Z. Liu, J. Liu and J. E. Saavedra, Effects of JS-K, a novel anti-cancer nitric oxide prodrug, on gene expression in human hepatoma Hep3B cells, Biomed. Pharmacother., 2017, 88, 367–373 CrossRef CAS PubMed.
- J. Ren, Y. Zheng, Z. Lin, X. Han and W. Liao, Macroporous resin purification and characterization of flavonoids from Platycladus orientalis (L.) Franco and their effects on macrophage inflammatory response, Food Funct., 2017, 8, 86–95 CAS.
- M. Wang, X. B. Yang, J. W. Zhao, C. J. Lu and W. Zhu, Structural characterization and macrophage immunomodulatory activity of a novel polysaccharide from Smilax glabra Roxb, Carbohydr. Polym., 2016, 156, 390–402 CrossRef PubMed.
- J. S. Lee, D. S. Kwon, K. R. Lee, J. M. Park, S. J. Ha and E. K. Hong, Mechanism of macrophage activation induced by polysaccharide from Cordyceps militaris culture broth, Carbohydr. Polym., 2015, 120, 29–37 CrossRef CAS PubMed.
- Z. J. Wang, D. H. Luo and Z. Y. Liang, Structure of polysaccharides from the fruiting body of Hericium erinaceus Pers, Carbohydr. Polym., 2004, 57, 241–247 CrossRef CAS.
- A. Q. Zhang, P. L. Sun, J. S. Zhang, C. H. Tang, J. M. Fan, X. M. Shi and Y. J. Pan, Structural investigation of a novel fucoglucogalactan isolated from the fruiting bodies of the fungus Hericium erinaceus, Food Chem., 2007, 104, 451–456 CrossRef CAS.
- Y. Zhang, M. Gu, K. P. Wang, Z. X. Chen, L. Q. Dai, J. Y. Liu and F. Zeng, Structure, chain conformation and antitumor activity of a novel polysaccharide from Lentinus edodes, Fitoterapia, 2010, 81, 1163–1170 CrossRef CAS PubMed.
- X. C. Liu, Z. Y. Zhu, Y. L. Tang, M. Wang, Z. Wang, A. J. Liu and Y. M. Zhang, Structural properties of polysaccharides from cultivated fruit bodies and mycelium of Cordyceps militaris, Carbohydr. Polym., 2016, 142, 63–72 CrossRef CAS PubMed.
- S. P. Nie, S. W. Cui, A. O. Phillips, M. Y. Xie, G. O. Phillips, S. Al-Assaf and X. L. Zhang, Elucidation of the structure of a bioactive hydrophilic polysaccharide from Cordyceps sinensis by methylation analysis and NMR spectroscopy, Carbohydr. Polym., 2011, 84, 894–899 CrossRef CAS.
- L. B. Ye, J. R. Li, J. S. Zhang and Y. J. Pan, NMR characterization for polysaccharide moiety of a glycopeptide, Fitoterapia, 2010, 81, 93–96 CrossRef CAS PubMed.
- Q. Guo, S. W. Cui, J. Kang, H. Ding, Q. Wang and C. Wang, Non-starch polysaccharides from American ginseng: physicochemical investigation and structural characterization, Food Hydrocolloids, 2015, 44, 320–327 CrossRef CAS.
- Y. Deng, L. X. Chen, B. X. Han, D. T. Wu, K. L. Cheong, N. F. Chen, J. Zhao and S. P. Li, Qualitative and quantitative analysis of specific polysaccharides in Dendrobium huoshanense, by using saccharide mapping and chromatographic methods, J. Pharm. Biomed. Anal., 2016, 129, 163–171 CrossRef CAS PubMed.
- Y. Zhang, T. Zhou, H. Wang, Z. Cui, F. Cheng and K. P. Wang, Structural characterization and in vitro antitumor activity of an acidic polysaccharide from Angelica sinensis (Oliv.) Diels, Carbohydr. Polym., 2016, 147, 401–408 CrossRef CAS PubMed.
- B. O. Petersen, I. C. Skovsted, B. S. Paulsen, A. R. Redondo and S. Meier, Structural determination of Streptococcus pneumoniae, repeat units in serotype 41A and 41F capsular polysaccharides to probe gene functions in the corresponding capsular biosynthetic loci, Carbohydr. Res., 2014, 400, 26–32 CrossRef CAS PubMed.
- P. Hu, Z. Li, M. Chen, Z. Sun, Y. Ling, J. Jiang and C. Huang, Structural elucidation and protective role of a polysaccharide from Sargassum fusiforme on ameliorating learning and memory deficiencies in mice, Carbohydr. Polym., 2016, 139, 150–158 CrossRef CAS PubMed.
- K. Marszewska, M. Czerwicka, S. J. Forsythe, K. Ossowska, H. Dziadziuszko and Z. Kaczyński, Structural studies of O-polysaccharide isolated from Cronobacter sakazakii, Sequence Type 12 from a case of neonatal necrotizing enterocolitis, Carbohydr. Res., 2015, 407, 55–58 CrossRef CAS PubMed.
- A. S. Shashkov, W. Zhang, A. V. Perepelov, A. Weintraub, B. Liu, G. Widmalm and Y. A. Knirel, Structure of the O-polysaccharide of Escherichia coli O132, Carbohydr. Res., 2016, 427, 44–47 CrossRef CAS PubMed.
- J. Jiang, F. Kong, N. Li, D. Zhang, C. Yan and H. Lv, Purification, structural characterization and in vitro antioxidant activity of a novel polysaccharide from Boshuzhi, Carbohydr. Polym., 2016, 147, 365 CrossRef CAS PubMed.
- W. Cui, G. Mazza and C. Biliaderis, Chemical structure, molecular size distributions, and rheological properties of flaxseed gum, J. Agric. Food Chem., 1994, 42, 1891–1895 CrossRef CAS.
- W. Wang, Y. Zou, Q. Li, R. Mao, X. Shao, D. Jin, D. Zheng, T. Zhao, H. Zhu and L. Zhang, Immunomodulatory effects of a polysaccharide purified from Lepidium meyenii Walp. on macrophages, Process Biochem., 2016, 51, 542–553 CrossRef CAS.
- L. A. O'neill and D. G. Hardie, Metabolism of inflammation limited by AMPK and pseudo-starvation, Nature, 2013, 493, 346–355 CrossRef PubMed.
- S. Gordon, Phagocytosis: an immunobiologic process, Immunity, 2016, 44, 463–475 CrossRef CAS PubMed.
- W. J. Wu, M. M. Zhang, Y. Ren, X. Cai, Z. Yin, X. Zhang, T. Min and H. Wu, Characterization and immunomodulatory activity of a novel peptide, ECFSTA, from Wheat Germ Globulin, J. Agric. Food Chem., 2017, 65, 5561–5569 CrossRef CAS PubMed.
- X. F. Xu, H. D. Yan and X. W. Zhang, Structure and immuno-stimulating activities of a new heteropolysaccharide from Lentinula edodes, J. Agric. Food Chem., 2012, 60, 11560–11566 CrossRef CAS PubMed.
- M. M. Zhang, W. J. Wu, R. Yao, X. F. Li, Y. Q. Tang, M. Tian, F. R. Lai and H. Wu, Structural characterization of a novel polysaccharide from Lepidium meyenii (maca) and analysis of its regulatory function in macrophage polarization in vitro, J. Agric. Food Chem., 2017, 65, 1146–1157 CrossRef CAS PubMed.
- A. Mantovani, A. Sica, S. Sozzani, P. Allavena, A. Vecchi and M. Locati, The chemokine system in diverse forms of macrophage activation and polarization, Trends Immunol., 2004, 25, 677–686 CrossRef CAS PubMed.
- S. Fuu, C. Pojung, H. Kuangyang, C. Kahlock, W. T. Huang, T. Chiaoyin, Y. F. Chen, H. C. Cheng and H. H. Chang, Purification, cloning, and functional characterization of a novel immunomodulatory protein from Antrodia camphorata, (bitter mushroom) that exhibits TLR2-dependent NF-κB activation and M1 polarization within murine macrophages, J. Agric. Food Chem., 2009, 57, 4130–4141 CrossRef PubMed.
- B. N. Gantner, R. M. Simmons, S. J. Canavera, S. Akira and D. M. Underhill, Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2, J. Exp. Med., 2003, 197, 1107–1117 CrossRef CAS PubMed.
- I. A. Schepetkin and M. T. Quinn, Botanical polysaccharides: macrophage immunomodulation and therapeutic potential, Int. Immunopharmacol., 2006, 6, 317–333 CrossRef CAS PubMed.
- K. Ito, A. Hirao, F. Arai, K. Takubo, S. Matsuoka, K. Miyamoto, M. Ohmura, K. Naka, K. Hosokawa and Y. Ikeda, Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells, Nat. Med., 2006, 12, 446–451 CrossRef CAS PubMed.
- Y. H. Hong, H. B. Peng, V. La Fata and J. K. Liao, Hydrogen peroxide-mediated transcriptional induction of macrophage colony-stimulating factor by TGF-beta1, J. Immunol., 1997, 159, 2418–2423 CAS.
- N. Kaul, R. Gopalakrishna, U. Gundimeda, J. Choi and H. J. Forman, Role of protein kinase C in basal and hydrogen peroxide-stimulated NF-κB activation in the murine macrophage J774A. 1 cell line, Arch. Biochem. Biophys., 1998, 350, 79–86 CrossRef CAS PubMed.
- Q. Yu, S. P. Nie, J. Q. Wang, P. F. Yin, D. F. Huang, W. J. Li and M. Y. Xie, Toll-like receptor 4-mediated ROS signaling pathway involved in Ganoderma atrum polysaccharide-induced tumor necrosis factor-α secretion during macrophage activation, Food Chem. Toxicol., 2014, 66, 14–22 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |