Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Influence of double (w1/o/w2) emulsion composition on lubrication properties
A. K. L.
Oppermann
 ab,
L. C.
Verkaaik
b,
M.
Stieger
a and
E.
Scholten
*b
ab,
L. C.
Verkaaik
b,
M.
Stieger
a and
E.
Scholten
*b
aDivision of Human Nutrition, Wageningen University & Research, P.O. Box 17, 6700 AA Wageningen, The Netherlands
bPhysics and Physical Chemistry of Foods, Wageningen University & Research, P.O. Box 17, 6700 AA Wageningen, The Netherlands. E-mail: elke.scholten@wur.nl; Tel: +31 317 482288
First published on 5th December 2016
Abstract
Double (w1/o/w2) emulsions are potential fat replacers in foods. Fats are known for their lubricating properties, which contribute to texture perception. It is therefore of interest to understand how the composition of double emulsions influences lubrication properties. This study focuses on the understanding of the influence of the fraction of inner dispersed aqueous phase w1 and the gelation of the w1 droplets on the lubrication properties of double emulsions. The addition of an inner water phase w1 to the oil droplets decreased friction at low entrainment speeds due to adsorption of the lipophilic emulsifier polyglycerol polyricinoleate (PGPR) at the hydrophobic tribo-surface. At higher entrainment speeds, double emulsions with w1 fractions of up to 6% (corresponding to fat reduction of 20%) displayed comparable tribological behavior as full-fat single (o/w2) emulsions. For double emulsions with gelled w1 droplets at higher w1 fractions of up to 15% (corresponding to fat reduction of 50%), an increase in friction was observed compared to full-fat single (o/w2) emulsions. The increase in friction is probably related to the presence of gelled droplets expelled from the inner w1 into the outer w2 phase, and to the deformability of (w1/o) droplets. Lubrication decreased when gelled particles were expelled from the inner w1 phase to the outer w2 phase. Lubrication also decreased when the deformability of (w1/o) droplets decreased, since less deformable (w1/o) droplets spread less easily on the tribo-pair surface. Knowledge about lubrication properties of double emulsions can be used in future studies to relate composition to sensory perception and develop double emulsions further as fat replacers.
1. Introduction
Emulsions and emulsion-based foods represent an interesting food category for fat reduction due to the often high amounts of dispersed fats and oils in those products. A potential approach for fat reduction is the use of double (w1/o/w2) emulsions. Double emulsions are complex multiphase systems, in which small water droplets are entrapped inside larger oil droplets (w1/o), which are subsequently dispersed in a water-continuous phase, w2. Consequently, at a given oil droplet size the oil droplet surface area remains similar to those of (o/w2) single emulsions, while the amount of oil is decreased.Oil droplets in emulsion-based foods contribute to the perception of certain sensory and mouthfeel attributes, such as creaminess and fattiness. Sensory perception of emulsions is influenced by rheological and tribological properties during different stages of oral processing.1,2 The amount of fat in an emulsion can greatly affect the lubrication of oral surfaces during consumption, and can therefore have a positive influence on sensory perception.
Several authors studied the lubrication properties of single oil-in-water (o/w2) emulsions. Generally, increasing oil content of single (o/w2) emulsions decreased friction coefficients by reducing close contact between the surfaces of the tribo-pair.3–10 However, when oil content in single emulsions exceeded a certain level (15 to 30%), no differences in lubrication behavior were found anymore between emulsions varying in oil volume fraction.6 It was suggested that friction is mostly affected by oil volume fraction at lower oil volume fractions, i.e. below 15%. Oil droplets are deformable and can provide lubrication between surfaces by forming a film or oil film patches. Furthermore, the solid fat content of oils has been shown to influence lubrication. In emulsion-filled gels, Liu and co-workers11 found that friction decreased with increasing solid fat content (SFC) due to partial coalescence of fat crystals, even though fat droplets with higher SFC are less deformable. Oil droplet size of emulsions also has been shown to influence friction. De Wijk and co-workers12 showed that increasing oil droplet size (up to 6 μm) in mayonnaise increased friction, indicating that smaller droplets have a larger effect on lubrication. Even though the effect of fat content of single emulsions on lubrication properties has been studied, the effect of emulsifier type on lubrication properties of single emulsions received less attention. It has been reported that low molecular weight surfactants such as sucrose esters or Tween 20 decrease friction in single emulsions with high oil content.2,13 Overall, the composition of single (o/w2) emulsions (fat content, oil droplet size, type of emulsifier) have an influence on the lubrication behavior.
While the lubrication properties of single emulsions have been studied, to the best of our knowledge the lubrication properties of double (w1/o/w2) emulsions have not been investigated. Double emulsions differ in various aspects from single emulsions. First, oil droplets in double emulsions are typically larger than in single emulsions to retain water droplets inside. While the diameter of oil droplets in single emulsions is typically of the order of 1–10 μm, the diameter of oil droplets in double emulsions is typically larger than 50 μm. Second, small water droplets, typically smaller than 1 μm, are dispersed into larger oil droplets. The inclusion of water droplets increases the viscosity of the oil droplets, and consequently decreases the deformability of the oil droplets. Third, double emulsions are prepared with two surfactants. The inner water droplets are stabilized by a lipophilic surfactant, often polyglycerol polyricinoleate (PGPR), and the outer phase usually by proteins such as whey protein isolate (WPI) or Na-caseinate. To increase the stability of double emulsions, the inner water droplets w1 can be gelled, which results in the retention of w1 droplets inside the oil droplets. Not much is known about the lubrication behavior of complex multiphase emulsions and how the lubrication properties are affected by their composition.
The aim of this study was to investigate the tribological properties of double (w1/o/w2) emulsions varying in composition. Specifically, the influence of inner dispersed water phase w1 fraction, dispersed oil phase fraction, and the effect of lipophilic emulsifier PGPR on the tribological properties were studied. Knowledge about lubrication properties of double emulsions can be used in future studies to relate emulsion composition to sensory perception and develop double emulsions as effective fat replacers.
2. Materials and methods
2.1 Materials
Whey protein isolate (WPI, BiPro JE-099-2-420) from Davisco Foods International Inc. (Le Sueur, MN) was used as hydrophilic emulsifier. The composition of WPI as stated by the manufacturer was 97.7% protein, 0.3% fat, 1.8% ash (dry weight basis). NaCl was obtained from Sigma-Aldrich (purity ≥99.5%, Sigma-Aldrich, Steinheim, Germany). Gelatin (type A, Type 250 PS 30) from Rousselot (Gent, Belgium) was used. Commercial sunflower oil was purchased from a local retailer (Wageningen, the Netherlands). Polyglycerol polyricinoleate (PGPR 4175) was kindly provided by Palsgaard (Juelsminde, Denmark). For all aqueous solutions, purified water (Milli-Q, 18.2 MΩ cm at 25 °C) was used.2.2 Preparation of emulsions
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 60 s. As reported previously,15 the maximum inner dispersed phase content was 30 wt%. To achieve higher fat reduction levels of 40 and 50 wt%, gelation of the inner water droplets was necessary. For double emulsions with gelatin in the inner aqueous phase, both the oil phase and the gelatin solution were heated to 60 °C in a water bath prior to mixing. A premixing step was used to add 40 or 50 wt% of the heated gelatin solution to the oil phase while increasing the mixing speed of a high speed blender (Ultra Turrax T25 with the dispersing tool S25-N 18G, IKA, Staufen, Germany) from 3000 rpm to 8000 rpm within 1 min and mixed at 8000 rpm for 4 min. The primary emulsion was then transferred to the Waring blender and mixed at 22
000 rpm for 60 s. As reported previously,15 the maximum inner dispersed phase content was 30 wt%. To achieve higher fat reduction levels of 40 and 50 wt%, gelation of the inner water droplets was necessary. For double emulsions with gelatin in the inner aqueous phase, both the oil phase and the gelatin solution were heated to 60 °C in a water bath prior to mixing. A premixing step was used to add 40 or 50 wt% of the heated gelatin solution to the oil phase while increasing the mixing speed of a high speed blender (Ultra Turrax T25 with the dispersing tool S25-N 18G, IKA, Staufen, Germany) from 3000 rpm to 8000 rpm within 1 min and mixed at 8000 rpm for 4 min. The primary emulsion was then transferred to the Waring blender and mixed at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 60 s. Samples were subsequently cooled under tap water for at least 15 min to allow gelation of the inner water droplets. Water droplet sizes (d0.5) of the different primary (w1/o) emulsions were similar and ranged from 150–250 nm with all droplets being smaller than 1 μm (Mastersizer 2000, Malvern Instruments, Worcestershire, UK) as reported previously.14,15
000 rpm for 60 s. Samples were subsequently cooled under tap water for at least 15 min to allow gelation of the inner water droplets. Water droplet sizes (d0.5) of the different primary (w1/o) emulsions were similar and ranged from 150–250 nm with all droplets being smaller than 1 μm (Mastersizer 2000, Malvern Instruments, Worcestershire, UK) as reported previously.14,15
Single oil-in-water (o/w2) emulsions were prepared by dispersing 30 wt% of oil phase (with or without PGPR) in 70 wt% of outer water phase w2 within one minute in a high speed blender (Ultra Turrax T25 with the dispersing tool S25-N 18G, IKA, Staufen, Germany), while increasing the speed from 3000 rpm to the required speed, after which mixing was continued for 4 min.
For some single (o/w2) emulsions, gelled particles (with gelatin as a gelling agent) were added to the outer water phase w2, to investigate the effect of gelled water droplets expelled from the inner w1 to the outer w2 water phase. To prepare the gelled particles, a 10 wt% water-in-oil (w1/o) emulsion containing a 10 wt% gelatin solution was prepared as described in section 2.2.2. The (w1/o) emulsion was centrifuged for 90 min at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (74
000 rpm (74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 rcf) at 15 °C using a high performance centrifuge (Avanti J-26 XP, rotor JA-25.50, Beckham Coulter, Brea, US) to separate the gelled particles from the oil phase. The pellet of gelled particles was washed with a 1 wt% WPI solution at a ratio of 1
600 rcf) at 15 °C using a high performance centrifuge (Avanti J-26 XP, rotor JA-25.50, Beckham Coulter, Brea, US) to separate the gelled particles from the oil phase. The pellet of gelled particles was washed with a 1 wt% WPI solution at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and the mixture was mixed using a high speed blender (Ultra Turrax T25 with the dispersing tool S25-N 18G, IKA, Staufen, Germany) for 5 min at 1200 rpm. WPI was added to aid the removal of the oil and the excess emulsifier. The mixture was then homogenized (Lab-scale homogenizer, Delta instruments, Drachten, the Netherlands) for six times at 150 bar. The sample was then centrifuged again under the same conditions for 60 min. The pellet was removed after this second centrifugation step. Fractions of this pellet (2.5 and 5 wt%) were added to single (o/w2) emulsions to investigate the effect of the presence of particles in the outer water phase w2.
2 and the mixture was mixed using a high speed blender (Ultra Turrax T25 with the dispersing tool S25-N 18G, IKA, Staufen, Germany) for 5 min at 1200 rpm. WPI was added to aid the removal of the oil and the excess emulsifier. The mixture was then homogenized (Lab-scale homogenizer, Delta instruments, Drachten, the Netherlands) for six times at 150 bar. The sample was then centrifuged again under the same conditions for 60 min. The pellet was removed after this second centrifugation step. Fractions of this pellet (2.5 and 5 wt%) were added to single (o/w2) emulsions to investigate the effect of the presence of particles in the outer water phase w2.
An overview of the composition and characteristics of all single (o/w2) and double (w1/o/w2) emulsions can be found in Table 1. Sample names indicate the emulsion type (“OW” for single emulsions, “WOW” for double emulsions), the amount of inner aqueous phase w1, oil phase and outer water phase w2 in the final emulsion, followed by the physical state of the inner aqueous phase (“NG” for non-gelled, “G” for gelled). For example, the label “WOW 3-27-70 NG” refers to a double emulsion containing 3 wt% of non-gelled w1 droplets dispersed in 27 wt% of oil, further dispersed in 70 wt% of outer continuous water phase w2. The 3 wt% of inner aqueous phase w1 and 27 wt% of oil correspond to a total of 30 wt% of dispersed (w1/o) phase. Since a small fraction of the inner water droplets is expelled to the outer water phase during preparation, the real mass fractions of the two aqueous phases are slightly different. The real dispersed phase (w1/o) fractions were determined by measuring the yield, i.e. the amount of w1 droplets remaining inside the oil droplets after emulsion preparation (see section 2.3.4). The real dispersed phase mass fractions are listed in Table 1.
| Double emulsionsa | PGPR in oil [wt%] | PGPR in w1/o/w2 [wt%] | Theoretical fat reduction [wt%] | Theoretical Φ(w1) in w1/o/w2 [wt%] | Yield [%] | Real Φ(w1) in w1/o/w2 [wt%] | Real composition w1-o-w2 [wt%] | Gelled particles in w2 [wt%] | Viscosity w1/o [mPa s] | Viscosity w1/o/w2 [mPa s] |
|---|---|---|---|---|---|---|---|---|---|---|
| WOW 3-27-70 NG | 1.8 | 0.486 | 10 | 3 | 44.8 ± 4.3 | 1.3 ± 0.04 | 1.3-27-71.7 | — | 84.1 | 7.2 ± 0.7 |
| WOW 6-24-70 NG | 2.7 | 0.648 | 20 | 6 | 62.5 ± 1.7 | 3.7 ± 0.02 | 3.7-24-72.3 | — | 121.0 | 5.5 ± 0.4 |
| WOW 9-21-70 NG | 4 | 0.840 | 30 | 9 | 46.7 ± 2.3 | 4.2 ± 0.02 | 4.2-21-74.8 | — | 189.7 | 4.0 ± 0.7 |
| WOW 3-27-70 G | 1.8 | 0.486 | 10 | 3 | 59.7 ± 3.7 | 1.8 ± 0.04 | 1.8-27-71.2 | 1.6 ± 0.2 | 84.1 | 6.8 ± 0.5 |
| WOW 6-24-70 G | 2.7 | 0.648 | 20 | 6 | 99.3 ± 2.0 | 6.0 ± 0.02 | 6.0-24-70.0 | 0.1 ± 0.2 | 117.0 | 5.1 ± 0.3 |
| WOW 9-21-70 G | 4 | 0.840 | 30 | 9 | 69.7 ± 0.3 | 6.3 ± 0.01 | 6.3-21-72.7 | 3.7 ± 0.0 | 192.5 | 5.3 ± 0.3 |
| WOW 12-18-70 G | 6 | 1.08 | 40 | 12 | 71.9 ± 1.0 | 8.6 ± 0.01 | 8.6-18-73.4 | 4.6 ± 0.2 | 346.5 | 3.8 ± 0.4 |
| WOW 15-15-70 G | 9 | 1.35 | 50 | 15 | 77.4 ± 0.9 | 11.6 ± 0.01 | 11.6-15-73.4 | 4.6 ± 0.2 | 800.0 | 3.7 ± 0.4 |
| Single emulsions | PGPR in oil [wt%] | PGPR in o/w2 [wt%] | Gelled particles in w2 [wt%] | Viscosity oil [mPa s] | Viscosity o/w2 [mPa s] |
|---|---|---|---|---|---|
| a In all double emulsions with a gelled inner water phase, the inner water phase contained 10 wt% gelatin. b Some PGPR might have been left around gelled particles added to w2. c 2.5 and 5 wt% are fractions of the pellet and do not consist 100% of gelled particles due to maximum packing of particles. d Not measured. | |||||
| OW 30-70 | 0 | 0 | — | 60.4 | 9.2 ± 1.0 |
| OW 30-70 0.1% PGPR | 0.1 | 0.03 | — | 8.7 ± 0.3 | |
| OW 30-70 0.2% PGPR | 0.2 | 0.06 | — | 6.2 ± 0.6 | |
| OW 30-70 0.5% PGPR | 0.5 | 0.15 | — | 7.0 ± 0.5 | |
| OW 30-70 1% PGPR | 1 | 0.3 | — | 6.4 ± 0.8 | |
| OW 30-70 2% PGPR | 2 | 0.6 | — | 8.4 ± 0.8 | |
| OW 30-70 4.5% PGPR | 4.5 | 1.35 | — | 6.6 ± 0.1 | |
| OW 30-70 + 2.5% gelled particles in w2 | 0 | 0b | 2.5 | ||
| OW 30-70 + 5% gelled particles in w2 | 0 | 0b | 5 | ||
2.3 Emulsion characterization
Based on the yield, real inner dispersed phase fractions φw1, real (weight-based) were calculated:
| φw1, real = yield × φw1, theoretical | (1) |
In case of non-gelled w1 droplets, coalescence of those water droplets with the outer water phase only leads to a slight decrease in the dispersed phase fraction. The loss of gelled w1 droplets, however, leads to the presence of gelled particles in the outer water phase, as shown in Table 1. Due to these changes during the emulsion preparation, the real fractions for both the inner water droplets and the outer water phase slightly deviate from the theoretical fractions. The real weight fractions are given in Table 1.
3. Results and discussion
3.1 Influence of inner water phase fraction on lubrication behavior of double emulsions
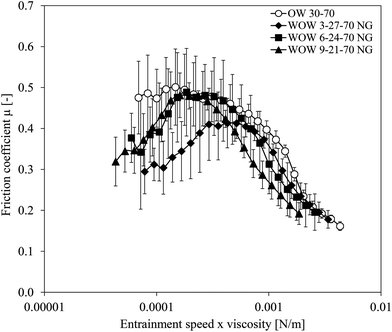
To investigate the influence of inner water droplets w1 within the oil droplets of the double (w1/o/w2) emulsions on lubrication properties, friction coefficients of double emulsions containing various amounts of inner water phase fractions are shown in Fig. 2. Fat reduction was varied by replacing 10, 20, or 30 wt% of the oil with small water droplets. Since the w1 droplets were not gelled, the maximum possible amount of added droplets is limited. For comparison, the friction coefficient of the corresponding full-fat single (o/w2) emulsion is shown in the same figure. All emulsions had a total dispersed (w1/o) fraction of 30 wt%.As can be seen in Fig. 2, all double emulsions were characterized by low friction coefficients at low entrainment speeds, followed by an increase in friction coefficient up to an inflection point before friction coefficients decreased again. First, we discuss the differences between the friction curves of all double emulsions, followed by a discussion of those curves in comparison to the single (o/w2) emulsion. For double emulsions with higher inner water phase fractions (6 and 9 wt% of the double emulsion, corresponding to 20 and 30 wt% fat reduction†), the increase in friction coefficient was more pronounced. Those double emulsions had slightly higher friction coefficients than the double emulsion with 3% inner water phase (corresponding to 10% fat reduction). We do not have an explanation for the lower friction at very low entrainment speeds of the double emulsion with 3 wt% inner water phase. Even though error bars are large, it seems that a higher inner water phase fraction tended to lead to an increase in friction. The increased number of small water droplets increased the viscosity of the dispersed (w1/o) droplets, resulting in less deformable (w1/o) droplets compared to when less water droplets were present in the oil. When dispersed (w1/o) droplets are less deformable, they cannot enter the gap easily and spread less easily on the tribo-surfaces to provide lubrication. Consequently, an increased friction was observed with increasing inner water phase fraction. The effect of an increased friction by an increase in particle stiffness has been described before.9 As the entrainment speed increased, dispersed (w1/o) droplets might enter the gap, and the surfaces start to part. Depending on the deformability of the dispersed (w1/o) droplets, the gap size between the two surfaces would be high for non-deformable droplets, or lower for deformable droplets. For higher deformability, the droplets may form a viscous film or patches of oil film. In this case, the droplets will become effective at reducing the friction coefficient.13 This might explain the reduction in friction at high entrainment speeds.
The increase in friction coefficient in the intermediate entrainment region has also been observed in other studies of emulsion-based foods.10,16 Baier and co-workers16 explained this increase in friction by an exclusion of the sample (low fat milk) from the contact zones. As a consequence, the sample cannot act as a lubricant. They observed this increase in friction particularly in samples with a very low fat content (<2%), while at higher fat contents, this increase was not found or was less pronounced. In the present study, the amount of dispersed oil phase was always 30% and therefore contradicts the hypothesis that the increased friction and presence of an inflection point is related to insufficient lubrication due to limited oil entering the gap. The high amount of dispersed phase in our study should be sufficient to provide lubrication. However, the increase in friction may not be related to the amount of the droplets, but the size of the oil droplets. In our study, the oil droplets had diameters (d0.5) of about 50 μm, and in combination with double emulsion droplets being less deformable than pure oil droplets, droplets at first might not have been able to enter the gap at low entrainment speeds since the gap in the boundary regime is expected to be 1 μm or smaller.17 Instead, the continuous water phase w2, known to be a poor lubricant, might have entered the gap, thereby increasing the friction coefficient. A similar explanation was postulated by Selway and Stokes17 who interpreted an increase in friction coefficient of a low-fat thickened cream as an indication that oil was not fully entrained in the contact zone and hence lubrication was dominated by the aqueous phase. An alternative hypothesis is that the oil droplets did enter the gap, but based on their low deformability at low speeds, friction increased since only a small part of their surfaces was in contact with the tribo-surfaces.
The single (o/w2) emulsions displayed higher friction coefficients than all double emulsions at low entrainment speeds. At higher entrainment speeds, double (w1/o/w2) emulsions and (o/w2) single emulsions had similar friction coefficients, even though no maximum was found for the single emulsion. At low entrainment speeds, friction is dominated by physical contact of the surfaces, and this regime is known as the boundary regime. In this regime, oil droplets cannot enter the gap and therefore cannot influence friction. At low entrainment speeds, double emulsions had the ability to decrease the friction coefficient in the boundary regime in contrast to single (o/w2) emulsions. In comparison to the single (o/w2) emulsion, double emulsions must contain ingredients other than oil droplets that lead to a decreased friction in the boundary regime.
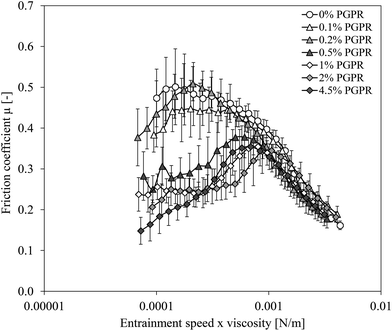
To explain this effect, we have to consider that water droplets inside the oil droplets have to be stabilized by a lipophilic surfactant. The lipophilic surfactant used in the present study is polyglycerol polyricinoleate (PGPR). An excess of PGPR was used to create small water droplets. PGPR can thus also be present in the continuous oil phase or as micelles in the outer water phase. As PGPR molecules are small and hydrophobic, they can easily enter the gap, adsorb on the hydrophobic PDMS surfaces and provide lubrication. To investigate the effect of PGPR present in the oil or continuous phase on lubrication, a series of single (o/w2) emulsions differing in PGPR concentrations (0–4.5% based on oil phase) were prepared (Table 1 and Fig. 3). Since PGPR lowers the (o/w2) interfacial tension,18 mixing speeds during emulsion preparation were adapted, so that oil droplet sizes of all (o/w2) emulsions were similar (50 μm). In this way, we investigate solely the effect of PGPR addition on lubrication.
As can be seen in Fig. 3, the addition of PGPR tended to decrease the friction coefficient of the single (o/w2) emulsion up to a concentration of 0.2 wt% at low entrainment speeds. When 0.5 wt% PGPR or more was present, the friction coefficient at low entrainment speeds decreased, and the friction coefficient was not further reduced upon an increase in lipophilic emulsifier concentration at 1 wt% and more. These results indicate that a critical emulsifier concentration was exceeded to decrease friction, since a sufficient concentration of emulsifier needs to be adsorbed at the PDMS surfaces to form an initial lubricating film. Our results contradict those of Douaire et al.19 who did not find a decrease in friction with increasing PGPR concentration for oil-continuous (w1/o) emulsions. This can be explained by the fact that their concentrations were much higher (1–7 wt%), indicating an excess of PGPR. The increase in lubrication found in the present study can be related to adhesion of emulsifier molecules to the tribological surfaces, which has also been observed by others.2,13 Liu et al.13 and Bellamy et al.2 showed that the type of emulsifier (WPI or Tween 20 for Liu and co-workers, and Na-caseinate or sucrose ester for Bellamy and co-workers) influenced lubrication properties, and that different emulsifiers interact differently with the surfaces. Since PDMS pins used in the present study are hydrophobic, the hydrophobic emulsifier PGPR might have adsorbed at the pin surfaces through hydrophobic interactions, thereby providing lubrication.
An inflection point of the friction coefficient was found when 0.5 wt% PGPR or more was added to the oil phase of the single emulsions (Fig. 3). In the boundary regime at low entrainment speeds, friction coefficients were low, followed by an increase in friction coefficient up to a maximum of about 0.35. At higher entrainment speeds, the effect of PGPR decreased and friction coefficients decreased to similar values as the other (o/w2) emulsions with lower PGPR concentrations. Cambiella et al.20 also studied the effect of type (nonionic, cationic, anionic) and concentration of hydrophilic emulsifiers on lubricating properties of single (o/w2) emulsions and found that especially at higher emulsifier concentrations, the film thickness between the two surfaces first increased with increasing entrainment speed, followed by a collapse to a very thin film occurring at entrainment speeds around 10 mm s−1. Formation (and collapse) of an emulsifier film could also play a role in the emulsions studied here, since PGPR is very lipophilic and can potentially adsorb at the hydrophobic PDMS surfaces, thereby reducing friction.
With regard to double (w1/o/w2) emulsions, it is most likely that the adsorption of PGPR onto the PDMS surface of the tribo-pair caused the decrease in friction coefficient at low entrainment speeds, while the entering of water-containing oil droplets into the gap probably explains the lubrication behavior at higher entrainment speeds.
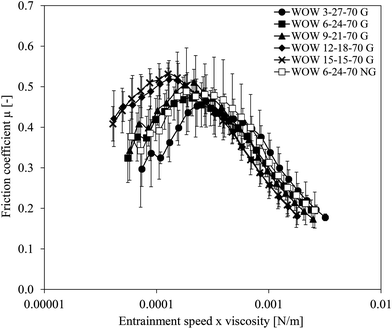
3.2 Effect of gelation of inner water droplets w1 on friction of double emulsions
The results in the previous section indicated that the amount of inner water droplets in double emulsions affects the lubrication behavior. However, the amount of inner w1 droplets that can be dispersed in oil is limited to 30%. To investigate the effect of higher fractions of inner water phase, gelation of those water droplets was necessary.14 By gelling the inner water droplets, we were able to obtain inner water fractions of up to 50%. In Fig. 4, the friction coefficient of double emulsions containing gelled droplets (closed symbols) between 3 and 15% (corresponding to fat reduction levels between 10 and 50%), as well as the friction coefficient of a double emulsion with a non-gelled inner water phase (open symbols), are plotted versus the entrainment speed × viscosity.Double emulsions with a similar fraction of non-gelled and gelled inner water phase (WOW 6-24-70 NG and WOW 6-24-70 G) showed very similar friction coefficients at all entrainment speeds. We therefore suggest that gelation of water droplets had a minor influence on the lubrication properties and the deformability of the oil droplets at this inner water fraction. In fact, the viscosity of the two corresponding primary (w1/o) emulsions was nearly identical (Table 1).
Even though error bars are large, friction coefficients generally tended to increase upon an increase of the gelled w1 droplet fraction. The increase of the gelled w1 droplet fraction leads to an increase of viscosity of the dispersed (w1/o) phase (Table 1), particularly when approaching the maximum packing fraction. We hypothesize that this increase in viscosity of the (w1/o) emulsion in turn leads to a decrease in deformability of the dispersed (w1/o) droplets. Additionally, this increase in viscosity of the dispersed (w1/o) phase also leads to an increase in viscosity ratio between the dispersed (w1/o) and continuous (w2) phase. The viscosity ratio also influences the deformability of the droplets. The smaller the viscosity differences between the oil droplets and the continuous phase, the more easily the oil droplets elongate, and the faster they are broken up.21–23 When viscosity differences between dispersed and continuous phase are large, i.e. the oil droplets being less deformable, oil droplets elongate less, and less film formation occurs. The lower deformability of the dispersed (w1/o) droplets increased the friction once they entered the gap and separated the two surfaces. De Vicente et al.24 indeed showed that the lubrication behavior of (o/w2) emulsions depended on the deformability of the dispersed phase. They showed that less deformable oil droplets could enter the gap between the tribo-surfaces. When large oil droplets did not contain inner water droplets, the droplet viscosity was low and the droplets were thus very deformable. In this case, the droplets may have formed a thin film at the inlet between the two surfaces, and then entered the gap. With increasing inner water phase fraction, the viscosity of the oil droplets increased and deformability decreased, which might have resulted in two effects. Either the decreased deformability increasingly obstructed the oil droplets from entering the contact zone between the two surfaces, or the less deformed oil droplets entered the gap, thereby increasing the film thickness, resulting in a higher friction coefficient. In the case that the droplets would not have entered the gap, the friction coefficient would have remained constant, and therefore this scenario is not likely to have happened in our systems.
Another possible reason for the increase in friction coefficient with increasing concentrations of gelled water droplets in the oil droplets might have been related to the amount of gelled water droplets that are lost during emulsion preparation. In the emulsions studied here, between 0 to 40% of the water droplets were expelled to the outer water phase, which resulted in fractions between 0.1 and 4.6 wt% of gelled particles in the outer water phase w2. Inside the oil droplets, those gelled droplets had an average size of 0.2 μm. When expelled to the outer phase, we expect our gelled droplets in the outer water phase to be of similar size, although they may have slightly swollen based on osmotic pressure differences. To investigate whether the presence of small gelled particles has an effect on the lubrication behavior and can explain some of the results observed, we prepared single (o/w2) emulsions and added a known fraction of gelled particles to the outer water phase. In this way, we investigate the influence of gelled particles in the continuous water phase solely.
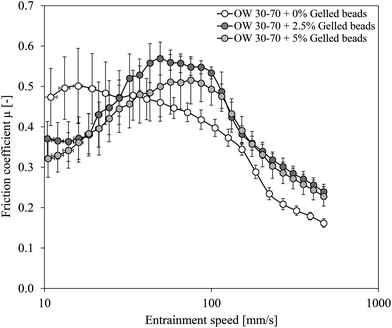
Since all single (o/w2) emulsions contained the same amount of dispersed oil phase with similar oil droplet sizes, differences in friction coefficient as depicted in Fig. 5 were related to gelled w1 beads present in the outer water phase w2. When gelled particles were added to the outer water phase, friction coefficients were lower at low entrainment speeds, but exceeded those of the single (o/w2) emulsion without gelled particles at increasing entrainment speeds. The initial decrease in friction coefficient was probably linked to remaining PGPR that was introduced in the emulsion system with the gelled particles. It is possible that some PGPR around the gelled particles was not completely removed by the washing step during preparation of the gelled particles. Another possibility is that gelled particles entered the gap already at very low entrainment speeds and acted as ball-bearing lubricants,13 as the gelled particles were comparable in size and shape to the microparticulated whey protein used by Liu and co-workers. At higher entrainment speeds, the friction coefficients of those single (o/w2) emulsions containing gelled particles in w2 exceeded those of emulsions without gelled particles. At these higher entrainments speeds, we assume that oil droplets were present in the gap, and that the presence of gelled particles increased the friction even further. However, for an increase in the concentration of the gelled particles, no increase in friction was found.
With regard to the increase in friction coefficient observed in double emulsions at intermediate entrainment speeds (Fig. 4), three explanations are possible based on the observations in Fig. 5. First, as shown in Fig. 5, gelled particles in w2 increase the friction coefficient at intermediate entrainment speeds. Since double emulsions with a larger fraction of gelled w1 beads also had higher fractions of expelled gelled w1 beads, the effect could be attributed to the presence of the gelled particles. In this case, we would expect a further increase in friction with increasing concentration of gelled particles in the outer water phase (Fig. 5). However, since we did not observe this, the increased friction cannot be attributed to the gelled particles in w2 only. Also other factors like the deformability of the oil droplets have to be considered. When the viscosity of the dispersed (w1/o) phase is increased due to the addition of small water droplets, deformability of the oil droplets is reduced. As a second effect, we propose that this reduced deformability might lead to oil droplets entering the gap in a less deformed state, thereby suddenly increasing the film thickness and therewith increase the friction. With increasing amounts of (gelled) water droplets, deformability of the oil droplets is further reduced. Additionally, it is possible that gelled particles in the outer water phase reduce the deformability of oil droplets further through obstruction by surrounding gelled particles close to the surfaces at intermediate entrainment speeds. Third, an increase in friction might be related to a collapse of a previously formed emulsifier film of PGPR at the hydrophobic PDMS surface. Above a critical speed, this emulsifier film might collapse, leading to a decrease in film thickness, resulting in an increase in friction.
To summarize, it seems that the lubrication properties of double emulsions with a gelled inner water phase are influenced by a combination of gelled particles in the outer water phase and the deformability of the oil droplets at intermediate entrainment speeds.
3.3 Influence of deformation during tribological measurements on composition of double emulsions
During the consumption of foods, foods are compressed between the tongue and the palate in the mouth. The gap between the tongue and palate depends on the forces exerted, which have been suggested to be comparable to forces exerted during tribology measurements.25,26 The compression and shear forces during consumption can change the composition of foods, for example by releasing serum or oil upon compression.27 For double emulsions, we hypothesize that inner water droplets might be released from the oil phase or oil droplets could coalesce when shear forces are applied during a tribological measurement. The loss of inner water droplets may result in changes in oil droplet size and may depend on the fraction of inner water droplets. To test the hypothesis, we investigated the oil droplet size and the change in fraction of (gelled) water droplets remaining in the oil phase before and after a tribological experiment (Table 2). After emulsion preparation, double emulsions were sheared in the tribometer at speeds from 0.47 mm s−1 to 470 mm s−1 for 4.3 min, followed by a decrease from 470 mm s−1 to 0.47 mm s−1 in 4.3 min. Samples were removed from the tribometer afterwards and their oil droplet size and yield measured. Real inner water phase fractions were calculated based on the yield as described in section 2.3.4.| Emulsion | w1 theoretical (wt% of double emulsion) | Real w1 in w1/o/w2 [wt%] | Oil droplet size [μm] | ||||
|---|---|---|---|---|---|---|---|
| Before | After | Average relative change [%] | Before | After | Average relative change [%] | ||
| WOW 3-27-70 G | 10 | 1.8 ± 0.04 | 1.4 ± 0.05 | −22% | 51.5 ± 1.3 | 53.3 ± 1.8 | +3% |
| WOW 6-24-70 G | 20 | 6.0 ± 0.02 | 4.8 ± 0.02 | −20% | 50.8 ± 1.7 | 52.5 ± 1.0 | +3% |
| WOW 9-21-70 G | 30 | 6.3 ± 0.0 | 4.8 ± 0.04 | −24% | 48.6 ± 1.0 | 48.9 ± 1.3 | +1% |
| WOW 12-18-70 G | 40 | 8.6 ± 0.01 | 7.3 ± 0.03 | −15% | 51.9 ± 0.4 | 50.4 ± 0.6 | −3% |
| WOW 15-15-70 G | 50 | 11.6 ± 0.01 | 9.8 ± 0.01 | −16% | 54.3 ± 0.9 | 56.3 ± 0.7 | +4% |
| WOW 3-27-70 NG | 10 | 1.3 ± 0.04 | 1.2 ± 0.02 | −8% | 51.9 ± 0.5 | 52.8 ± 0.2 | +2% |
| WOW 6-24-70 NG | 20 | 3.8 ± 0.02 | 3.0 ± 0.03 | −21% | 51.8 ± 1.1 | 52.5 ± 0.5 | +1% |
| WOW 9-21-70 NG | 30 | 4.2 ± 0.02 | 3.0 ± 0.03 | −29% | 47.7 ± 1.3 | 50.2 ± 3.8 | +5% |
The size of the oil droplets of the double emulsions did not change considerably before and after the tribological experiment and tended to slightly increase by 1 to 5%, while the fraction of inner water droplets decreased considerably after the tribological experiment by up to 29%. This decrease in w1 fraction might have been due to the shear stresses occurring during the tribological measurement between the tribo-pair surfaces. Oil droplets that enter the gap are deformed, so that the (o/w2) interfacial area increases. This brings more water droplets into contact with the (o/w2) interface, increasing the probability of coalescence between the water droplets and the outer water phase and expulsion of the gelled droplets. Lower fractions of water droplets would lead to smaller oil droplet sizes. Additionally, high shear stresses between the two surfaces might actually break up the oil droplets. These two effects would lead to a decrease in the oil droplet size. Since a slight increase in droplet size was observed in our experiments, the results imply that also coalescence between the oil droplets occurred during the tribological measurement. Slow re-arrangements of whey proteins at the interface during the deformation might have increased the probability of oil droplet coalescence. We hypothesize that oil droplets broke up and coalesced during deformation so that overall the oil droplet size increased marginally. Comparison of oil droplet size distributions before and after friction measurements (rather than just comparing the averages) did not provide further insights since neither smaller oil droplets (indicating oil droplet breakup) nor larger oil droplets (indicating oil droplet coalescence) were observed.
These results show that the emulsion composition during deformation in a tribological measurement changed considerably due to the release of inner water droplets while oil droplet sizes only slightly increased. These changes might be formulation-dependent, and differ with concentration and types of emulsifiers, droplet sizes, and other characteristics.
3.4 General discussion
Several mechanisms may play a role in the lubrication properties of double emulsions. In general, we found that friction was decreased at low entrainment speeds, while friction increased with increasing (gelled) inner water phase fractions at intermediate entrainment speeds. At higher entrainment speeds, friction coefficients decreased for all double emulsions.Based on these observations, we hypothesize that different aspects of the double emulsions dominate the lubrication properties at different entrainment speeds. The influence of the emulsion composition is discussed below.
As illustrated in Fig. 6, the addition of PGPR in the emulsion leads to a decrease in friction at low entrainment speeds, probably by an adsorbed emulsifier film at the hydrophobic PDMS surface of the tribo-pair. With increasing entrainment speed, deformable oil droplets enter the gap and lubricate the tribo-surfaces by forming oil film patches. The addition of inner water droplets increases the viscosity of the dispersed (w1/o) droplets and thereby reduces the deformability, leading to an increased distance between the surfaces and increased friction at intermediate entrainment speeds. With increasing amounts of (gelled) water droplets, deformability of the oil droplets is further reduced. As a consequence, oil droplets provide less lubrication due to less spreading of the oil droplets between the surfaces. This results in increased friction at intermediate entrainment speeds. Gelled particles in the outer water phase increase friction in this regime even further, possibly by further hindering the movement of (w1/o) droplets. At higher entrainment speeds, the droplets become more deformed, form films and thereby decrease friction.
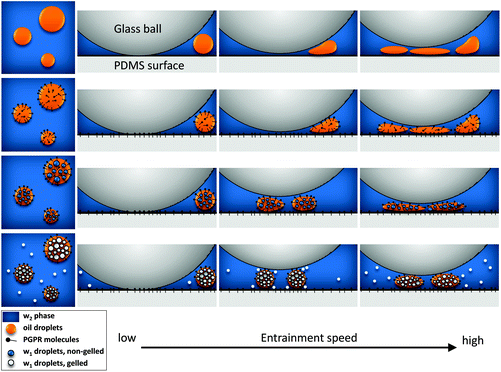 | ||
| Fig. 6 Schematic representation of proposed mechanisms contributing to lubrication properties of double emulsions. | ||
4. Conclusions
In the present study, we investigated the influence of composition on lubrication properties of double emulsions. This study focused on the effect of the fraction of inner dispersed aqueous droplets w1 and the gelation thereof on the lubrication properties of double emulsions to better understand the mechanisms underlying lubrication properties.The use of the lipophilic emulsifier PGPR reduced friction at low entrainment speeds probably due to PGPR adsorbing at the hydrophobic PDMS surfaces of the tribo-pair. Increasing fractions of inner water droplets in the oil droplets led to an increase in friction. This is probably due to an increase of the effective viscosity of the dispersed phase, independent of its exact composition. An additional increase in friction was observed in the presence of gelled particles in the outer water phase that hinder the deformation of the oil droplets. Interactions between oil, water, surfactants and the surfaces seem to further influence the lubrication mechanisms. Through these insights into lubrication behavior of double emulsions, advancements in the development of fat-reduced emulsions with similar sensory perception as full-fat emulsions can be achieved. Regarding future research in this field, the effect of temperature and saliva on lubricating properties of double emulsions are of interest to obtain results that are better comparable to in-mouth oral processing of liquids. The effect of temperature on the lubrication behavior of double emulsions with a gelled inner w1 phase using gelatin as the gelling agent is particularly of relevance as gelatin melts at body temperature and may therefore further influence lubrication. The bottom-up approach followed in the present study aiming to understand the role of particular components and phases in lubrication processes is important for the development of double emulsions as fat replacers.
Acknowledgements
The study received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement nr 289397 (TeRiFiQ project). The authors would like to thank Herman de Beukelaer and Willem Vogelzang for help with the DSC measurements.References
- G. A. van Aken, M. H. Vingerhoeds and R. A. de Wijk, Textural perception of liquid emulsions: Role of oil content, oil viscosity and emulsion viscosity, Food Hydrocolloids, 2011, 25(4), 789–796 CrossRef CAS.
- M. Bellamy, N. Godinot, S. Mischler, N. Martin and C. Hartmann, Influence of emulsion composition on lubrication capacity and texture perception, Int. J. Food Sci. Technol., 2009, 44(10), 1939–1949 CrossRef CAS.
- B. Le Calvé, C. Saint-Léger, R. Babas, J. L. Gelin, A. Parker and P. Erni, et al., Fat Perception: How Sensitive are We?, J. Texture Stud., 2015, 46(3), 200–211 CrossRef.
- R. A. de Wijk and J. F. Prinz, Mechanisms underlying the role of friction in oral texture, J. Texture Stud., 2006, 37, 413–427 CrossRef.
- H. S. Joyner, C. W. Pernell and C. R. Daubert, Impact of Oil-in-Water Emulsion Composition and Preparation Method on Emulsion Physical Properties and Friction Behaviors, Tribol. Lett., 2014, 56(1), 143–160 CrossRef CAS.
- M. E Malone, I. A. M. Appelqvist and I. T. Norton, Oral behaviour of food hydrocolloids and emulsions. Part 1. Lubrication and deposition considerations, Food Hydrocolloids, 2003, 17(6), 763–773 CrossRef CAS.
- A. Chojnicka-Paszun, H. H. J. de Jongh and C. G. de Kruif, Sensory perception and lubrication properties of milk: Influence of fat content, Int. Dairy J., 2012, 26(1), 15–22 CrossRef.
- R. A. de Wijk, J. F. Prinz and A. M. Janssen, Explaining perceived oral texture of starch-based custard desserts from standard and novel instrumental tests, Food Hydrocolloids, 2006, 20(1), 24–34 CrossRef CAS.
- S. Giasson, J. N. Israelachvilli and H. Yoshizawa, Thin film morphology and tribology study of mayonnaise, J. Food Sci., 1997, 62(4), 640–652 CrossRef CAS.
- A. Krzeminski, K. A. Prell, M. Busch-Stockfisch, J. Weiss and J. Hinrichs, Whey protein–pectin complexes as new texturising elements in fat-reduced yoghurt systems, Int. Dairy J., 2014, 36(2), 118–127 CrossRef CAS.
- K. Liu, M. Stieger, E. van der Linden and F. van de Velde, Fat droplet characteristics affect rheological, tribological and sensory properties of food gels, Food Hydrocolloids, 2015, 44(0), 244–259 CrossRef CAS.
- R. A. de Wijk and J. F. Prinz, The role of friction in perceived oral texture, Food Qual. Prefer., 2005, 16(2), 121–129 CrossRef.
- K. Liu, Y. Tian, M. Stieger, E. van der Linden and F. van de Velde, Evidence for ball-bearing mechanism of microparticulated whey protein as fat replacer in liquid and semi-solid multi-component model foods, Food Hydrocolloids, 2016, 52, 403–414 CrossRef CAS.
- A. K. L. Oppermann, M. Renssen, A. Schuch, M. Stieger and E. Scholten, Effect of gelation of inner dispersed phase on stability of (w1/o/w2) multiple emulsions, Food Hydrocolloids, 2015, 48, 17–26 CrossRef CAS.
- A. K. L. Oppermann, B. Piqueras-Fiszman, C. De Graaf, E. Scholten and M. Stieger, Descriptive sensory profiling of double emulsions with gelled and non-gelled inner water phase, Food Res. Int., 2016, 85, 215–223 CrossRef CAS.
- S. Baier, D. Elmore, B. Guthrie, T. Lindgren, S. Smith and A. Steinbach, A new tribology device for assessing mouthfeel attributes of foods, 5th International Symposium on Food Structure and Rheology, 2009 Search PubMed.
- N. Selway and J. R. Stokes, Insights into the dynamics of oral lubrication and mouthfeel using soft tribology: Differentiating semi-fluid foods with similar rheology, Food Res. Int., 2013, 54(1), 423–431 CrossRef.
- I. Gülseren and M. Corredig, Interactions at the interface between hydrophobic and hydrophilic emulsifiers: Polyglycerol polyricinoleate (PGPR) and milk proteins, studied by drop shape tensiometry, Food Hydrocolloids, 2012, 29(1), 193–198 CrossRef.
- M. Douaire, T. Stephenson and I. T. Norton, Soft tribology of oil-continuous emulsions, J. Food Eng., 2014, 139, 24–30 CrossRef.
- A. Cambiella, J. M. Benito, C. Pazos, J. Coca, M. Ratoi and H. A. Spikes, The effect of emulsifier concentration on the lubricating properties of oil-in-water emulsions, Tribol. Lett., 2006, 22(1), 53–65 CrossRef CAS.
- B. J. Bentley and L. G. Leal, An experimental investigation of drop deformation and breakup in steady, two-dimensional linear flows, J. Fluid Mech., 1986, 167, 241–283 CrossRef CAS.
- H. P. Grace, Dispersion phenomena in high viscosity immiscible fluid systems and application of static mixers as dispersion devices in such systems, Chem. Eng. Commun., 1982, 14, 225–277 CrossRef CAS.
- P. Stroeve and P. P. Varanasi, An experimental study on double emulsion drop breakup in uniform shear flow, J. Colloid Interface Sci., 1984, 99(2), 360–373 CrossRef CAS.
- J. de Vicente, H. A. Spikes and J. R. Stokes, Viscosity Ratio Effect in the Emulsion Lubrication of Soft EHL Contact, J. Tribol., 2006, 128(4), 795–800 CrossRef CAS.
- J. R. Stokes, M. W. Boehm and S. K. Baier, Oral processing, texture and mouthfeel: From rheology to tribology and beyond, Curr. Opin. Colloid Interface Sci., 2013, 18(4), 349–359 CrossRef CAS.
- J. Chen and J. R. Stokes, Rheology and tribology: Two distinctive regimes of food texture sensation, Trends Food Sci. Technol., 2012, 25(1), 4–12 CrossRef CAS.
- G. Sala, M. Stieger and F. van de Velde, Serum release boosts sweetness intensity in gels, Food Hydrocolloids, 2010, 24(5), 494–501 CrossRef CAS.
Footnote |
| † Due to loss of inner water droplets during emulsification, real w1 fractions were very similar (3.7 and 4.2 wt%). |
| This journal is © The Royal Society of Chemistry 2017 |