Thermoresponsive structured emulsions based on the fibrillar self-assembly of natural saponin glycyrrhizic acid†
Zhili
Wan
a,
Yingen
Sun
a,
Lulu
Ma
a,
Jian
Guo
a,
Jinmei
Wang
a,
Shouwei
Yin
a and
Xiaoquan
Yang
*ab
aResearch and Development Center of Food Proteins, Department of Food Science and Technology, South China University of Technology, Guangzhou 510640, China. E-mail: fexqyang@163.com; fexqyang@scut.edu.cn; Fax: +86 20-87114263; Tel: +86 20-87114262
bGuangdong Province Key Laboratory for Green Processing of Natural Products and Product Safety, South China University of Technology, Guangzhou 510640, China
First published on 21st November 2016
Abstract
We report the novel use of the naturally occurring saponin, glycyrrhizic acid (GA) as a structuring material to transform liquid oil into a soft-solid structured emulsion system. The GA nanofibrils from the anisotropic self-assembly of GA molecules were first used as stabilizers to fabricate olive oil-in-water emulsions using a facile one-step emulsification at high temperature. Then, the obtained emulsions were further self-organized into the emulsion gel by applying a subsequent cooling to trigger the gel network formation, which is mostly due to the enhanced noncovalent interactions among GA fibrils in the continuous phase as well as at the droplet surface. The GA fibrils could adsorb at the interface in a multilayer form, leading to the formation of unique fibril shells with high electrostatic repulsive force, which could provide superior stability for the GA fibril-stabilized oil droplets and thus the whole emulsion gel during storage and heating. The thermoreversible gel–sol transitions of a self-assembled GA fibrillar network in the continuous phase endow the stable emulsion gels with a temperature-responsive switchable behavior. Moreover, the GA fibril-coated oil droplets embedded in the network were found to be closely packed together and connected with the gel matrix. As a consequence, the emulsion gels exhibited many interesting rheological behaviors, including a high gel strength, shear sensitivity, and good thixotropic recovery. These simple and inexpensive smart responsive oil structuring materials based on natural saponins could find novel applications in the fields of food, pharmaceuticals, or cosmetics.
1. Introduction
In recent years, the development of liquid oil-based functional soft solids and complex fluids, such as oleogels (or organogels) and structured emulsions, has become a rapidly expanding area of research, in particular due to their potential practical applications in the field of pharmaceuticals as drug delivery vehicles,1,2 for oil structuring in foods,3–5 stability improvement in cosmetics,6,7 and lubricant engineering.8 For example, in food processing, due to the recent ban of trans-fats and evolving discussion on the safety of saturated fats, a lot of research efforts have been currently focused on adopting alternative strategies to structure edible liquid oils for trans and saturated fat replacement in food products.9 Generally, oleogel systems are often used as the structuring alternatives, which are created based on the supramolecular assembly of low molecular weight oleogelators such as 12-hydroxystearic acid,10,11 waxes,12,13 fatty acids or alcohols,14 lecithins,15 and phytosterol–oryzanol mixtures,16 or a few biopolymers, such as ethylcellulose and modified chitins.17–20 Another effective approach to structure liquid oil is through structured emulsions. In comparison with oleogels, more biopolymers like proteins can be used to generate structured emulsions (emulsion gel or gelled emulsion),21,22 whereas monoglycerides are the only commonly used small-molecule structuring agents for oil-in-water (O/W) emulsions.9,23 Given the enormous potential of structured oils in different fields, it is therefore still essential to identify natural, sustainable, and food-grade materials for liquid oil structuring.The self-assembly of small amphiphilic molecules, especially the naturally occurring molecules as building blocks for the construction of functional materials has received increasing attention in terms of bioavailability, biocompatibility, and biodegradability.24–29 These natural surface-active molecules, such as triterpenoid saponins, exhibit unique self-assembling properties and in most cases also show many biological activities for the human body, which make them particularly attractive for application in the food, cosmetic and pharmaceutical fields. Recently, increasing efforts have been made to use triterpenoid saponins like glycyrrhizic acid (GA) as structural building blocks to form supramolecular assemblies due to their multiple functional groups, rigid skeletons, and unique stacking behaviors.24,27,28,30 These features render triterpenoid saponins as one of the ideal candidates for the construction of unique supramolecular architectures via self-assembly, especially the supramolecular organogels.24–27 For example, Bag and coworkers reported that many triterpenoids such as GA and arjunolic acid can be used to create thermoreversible organogels in different organic solvents via the formation of self-assembled fibrillar networks.26,27 Lu et al. showed that the triterpenoid derivatives conjugated with peptides were also capable of self-assembling into supramolecular organogels in aromatic solvents through noncovalent intermolecular hydrogen bonding and van der Waals interactions.31
GA, a natural triterpenoid saponin, is considered to be the main ingredient of licorice root extract and is widely used in candies and sweets due to its intense sweetness (50 times sweeter than sucrose). GA also exhibits many biological effects, such as antiinflammatory, antitumour, antivirus and antifungal effects.32 Chemically, GA is a monodesmosidic saponin comprised of a hydrophobic triterpenoid aglycon moiety (18β-glycyrrhetinic acid) attached to a hydrophilic diglucuronic unit. Due to the amphiphilic structure, GA molecules show an expected hierarchical self-assembly behavior in aqueous solution.28,30,33 The anisotropic self-assembly of GA in water leads to the formation of long nanofibrils first, which can further form a fibrillar network structure upon increasing concentrations, and finally forms a supramolecular hydrogel.28 As mentioned earlier, GA can also assemble into organogels in different organic solvents.24,27 However, unfortunately, GA is unable to form supramolecular assemblies directly in vegetable oils due to its very limited solubility in oil, and as a consequence, no information is available about the applications of GA as a structurant in vegetable oil structuring. However, as an amphiphilic molecule, GA or its assemblies (fibrils) do show some affinity for the oil phase and thus can be used as natural emulsifiers/stabilizers. Based on the unique combination of gelation and emulsifying behaviors, it is reasonably speculated that GA may have great possibilities as a natural structuring agent for constructing a new type of structured emulsion system.
Here, we demonstrated for the first time the use of a natural saponin GA as novel building blocks to construct a vegetable oil structuring system (emulsion gels) based on the fibrillar self-assembly of GA molecules. Through a one-pot emulsification at high temperature (80 °C), an olive oil-in-water (O/W) emulsion stabilized by GA nanofibrils was first prepared, and the emulsion droplets showed a multilamellar and highly charged fibril shell, providing a high electrostatic stability during storage and heating. Then, a subsequent cooling procedure was applied to strengthen the noncovalent interactions between GA fibrils in the continuous phase as well as at the interface, triggering the fibrillar network formation, and finally the successful fabrication of the soft solid emulsion gels was achieved. We characterized the microstructure and rheological behaviors including oscillatory amplitude and frequency sweeps, flow, and thixotropic behaviors of the GA fibril-based emulsion gels as a function of GA fibril and oil phase concentrations. The stability and large deformation mechanical properties of emulsion gels were also investigated. The results are relevant for novel applications in e.g. foods, pharmaceuticals, or cosmetics.
2. Experimental section
2.1 Materials
Glycyrrhizic acid mono ammonium salt (GA, purity >98%) was purchased from Acros Organics, USA. Olive oil (Bellina, ExiomFOOD, Spain) was purchased from a local supermarket (Guangzhou, China) and purified with Florisil (60–100 mesh, Sigma Aldrich) to remove impurities as described elsewhere.33 Nile Red and Thioflavin T (ThT) were purchased from Sigma-Aldrich (St Louis, MO, USA). Millipore water (18.2 MΩ cm at 25 °C) was used throughout this work, and all other chemicals used were of analytical grade.2.2 Characterization of GA nanofibrils
2.3 Preparation of GA fibril-based emulsion gels
A stock solution of GA fibrils was prepared by dissolving appropriate amounts of GA in water in a sealed vial, and then heated at 80 °C in a water bath under mild agitation until a clear solution was obtained. O/W emulsions were prepared by first dispersing olive oil in GA fibril solutions, and the dispersion was then incubated at 80 °C in a water bath under mild agitation for 5 min. After that, the resulting samples were immediately sheared using an Ultra-Turrax T10 (IKA-Werke GmbH & Co., Germany) at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 min. The resulting emulsions were cooled down and then stored overnight (12 h) at room temperature (25 °C) before further experiments.
000 rpm for 2 min. The resulting emulsions were cooled down and then stored overnight (12 h) at room temperature (25 °C) before further experiments.
To investigate the effect of GA fibril concentrations on the emulsion gel formation, the oil fraction was constant at 60 wt% with varied GA fibril concentrations from 0.5 to 4 wt%. In addition, the samples with various oil fractions (10–60 wt%) were also prepared at a constant GA fibril concentration of 4 wt%. The drying stability of emulsion gels was achieved using lyophilization, where the samples were frozen at −40 °C, followed by drying for 24 h in a Christ DELTA 1-24 LSC freeze-dryer (Christ, Germany). To evaluate their capacity as carriers for functional ingredients, the emulsion gels were prepared similarly by dissolving β-carotene (0.1 wt% of oil) into the oil prior to the homogenization step.
2.4 Droplet size and surface charge measurements
The droplet size (surface area weighted mean diameter, d32) and zeta potential of the GA fibril-based emulsions were measured using a Mastersizer 3000 and Nano ZS Zetasizer (Malvern Instruments Ltd, UK), respectively, after appropriate dilution with water. The refractive indices of olive oil and water were taken as 1.467 and 1.330, respectively. All measurements were carried out at 25 °C, and the results reported are averages of three measurements.2.5 Microstructure observations
The microstructure of emulsions/emulsion gels was studied using a confocal laser scanning microscope (CLSM, Leica Microsystems Inc., Heidelberg, Germany) and a polarized light microscope (PLM, Axioskop 40 Pol/40A Pol, Zeiss, Göttingen, Germany) equipped with a Power Shot G5 camera (Canon, Japan) and a Liakam hot stage (CI 94). For PLM, the emulsions/emulsion gels were placed on a flat slide and covered by using a cover slip. The magnification was 500× (50 × 10), and each image was acquired under normal and polarized light. To record the change in the microstructure of gels during heating (25–80 °C) and cooling (80–25 °C) processes at a rate of 5 °C min−1, the samples were transferred onto the hot stage, and the microstructure was observed under polarized light and recorded every 5 °C.For CLSM, Nile Red (0.1 wt%), a fluorescent dye for oil, was first dissolved in olive oil which was then used to prepare the emulsion gels. The samples were placed on concave confocal microscope slides and covered with coverslips. They were examined using an argon krypton laser (ArKr, 488 nm) with a 100× oil immersion objective lens at room temperature (25 °C). To study the distribution of GA on the gels, ThT was used to label the GA fibrillar network. ThT (0.01 wt%) was first dissolved in GA solutions prior to the gel preparation. The 458 nm line of an argon laser was used to excite the samples, and the emission fluorescence was observed between 470 and 560 nm. The oil phase dyed with Nile Red is green, whereas the GA network dyed with ThT is blue.
2.6 Rheological measurements
The rheological measurements of emulsion gels were carried out on a Haake RS600 rheometer (Haake Co., Germany) equipped with a Universal Peltier system and water bath (MultiTemp III, Amersham Biosciences) for temperature control. A parallel plate geometry of 27.83 mm diameter was used, and the gap was set at 1.0 mm. A range of oscillatory experiments including amplitude sweeps (stress = 0.1–1000 Pa, frequency = 1 Hz) and frequency sweeps (0.1–100 rad s−1, stress = 1 Pa, within the linear viscoelastic region) were performed at 25 °C. Temperature sweeps including heating from 25 °C to 80 °C and cooling back to 25 °C at a rate of 2 °C min−1 were also carried out at a constant stress of 1 Pa and a frequency of 1 Hz.In addition to the oscillatory measurements, the flow measurements were performed at an increasing shear rate from 0.1 to 50 s−1. The thixotropic behavior was studied by measuring the viscosity of emulsion gels with time at alternating low and high shear rates (0.1 and 10 s−1, respectively). All the measurements were performed at 25 °C.
2.7 Large deformation mechanical test
The large deformation compression test of emulsion gels was performed using a Universal testing machine (Instron 5943, USA). Samples were penetrated by a cylindrical probe of 25 mm diameter to a depth of 50% of their original height at a rate of 10 mm s−1 with a 0.1 N trigger value. The displacement distance was calculated relative to the starting point for each sample and reported as the relative displacement. Since the force–displacement curves start when the instrument measures the first force value (>trigger force), the force–displacement curves for different samples had different starting points. All the measurements were performed at 25 °C.3. Results and discussion
As a naturally occurring chiral saponin, GA molecules can self-assemble in water into long nanofibrils with a thickness of around 2.5 nm, independently of GA concentration.28,30 Previous studies have demonstrated that at a concentration of 0.1 wt%, almost all of the GA monomers assembled to form uniform fibrils and only minimum detectable monomers remained in the solution phase.28 In the present work, it can be seen that at a concentration of 0.25 wt% the GA fibrils covered the whole surface of the mica substrate (Fig. 1Aa and Ab), and the GA fibril solution (0.25 wt%) started to become significantly viscous due to the interfibrillar junctions and entanglements.28,29 Due to the amphiphilic nature, GA monomers and their assemblies (fibrils) should have affinity for the oil phase, and thus decrease the interfacial tension effectively. As expected, it is seen from Fig. S1A (ESI†) that the interfacial tension of GA solutions (0.025–0.1 wt%) at the oil–water interface gradually decreased with the adsorption time (30 min), and the adsorption kinetics became faster with increasing GA concentration, indicating the ability of GA fibrils to reduce interfacial tension.3.1 Multilayer emulsions stabilized by GA fibrils
Herein, we further prepared an O/W emulsion by using 0.25% GA fibril as emulsifiers. From Fig. 1B, it can be seen that under the present conditions, the 0.25% GA fibril-stabilized emulsion (5% oil phase) showed a d32 at around 2.6 μm with a relatively homogeneous droplet size distribution. Interestingly, these emulsion droplets could remain stable after repeated heat treatments (80 °C, 20 min) and storage at room temperature for 60 days (Fig. 1B). We then studied the morphology of oil droplets by using PLM (Fig. 1B). A striking observation from PLM images was the presence of a so-called “Maltese cross” interference figure (Fig. 1B), which is the characteristic appearance of anisotropic materials with two vibration directions.34 Accordingly, the presence of a radiant halo with a Maltese cross strongly suggests the formation of a multilayer structure of the emulsion droplets’ shells. This result indicates that the GA fibrils may adsorb at the oil droplet surface in a multilayer form probably due to the interactions (hydrogen bonds) between fibrils, thus leading to the multilayer interfacial organization. Similar multilayer adsorption behavior is also observed at the interface stabilized by protein long fibrils.35,36The zeta potential result showed that the emulsion droplets (pH = 4.5) were highly negatively charged (−60.8 mV), which should be due to the multilayer adsorption of charged GA fibrils onto the droplet surface. This will provide high electrostatic forces for emulsion stabilization. From a rheological point of view, the surface-active agents with highly elastic adsorption layers at the oil–water interface are expected to produce stable emulsions.37 However, the dilatational rheological measurements showed that the interfacial layer formed from the GA fibril solution had a fairly viscous response in dilatation deformations (Fig. S1B, ESI†), and the surface elasticity values were also very low (around 5–8 mN m−1, 1.5–10% amplitude). Previous studies have also demonstrated that the GA fibril solution (0.5 wt%) exhibited a negligible surface elasticity and very low viscosity at the air–water interface during shear deformations.38 On the basis of these results, we thus conclude that the superior stability of emulsions during heating and storage (Fig. 1B) is mainly attributed to the formation of multilayer GA fibril shells with high electrostatic repulsive forces, which could effectively protect the emulsion droplets against flocculation and coalescence. These results indicate that the GA nanofibrils from the supramolecular self-assembly of GA molecules could be used as simple and effective building blocks to fabricate stable O/W emulsions.
3.2 Formation of GA fibril-based emulsion gels
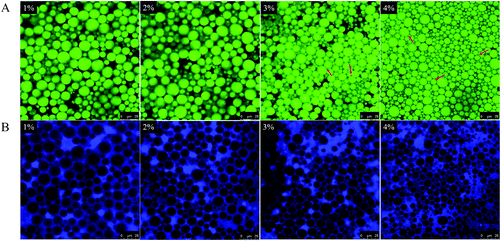
It has been demonstrated that the self-assembly of GA molecules in water leads to the formation of a fibrillar network structure and thus a hydrogel is formed through noncovalent interactions (hydrogen bonds) between GA fibrils.28,29,33 As shown in Fig. S2 (ESI†), a transparent hydrogel could be formed at the low GA concentration of 0.5 wt% due to the noncovalent junctions and entanglements between fibrils (Fig. S2A, ESI†), and the viscoelastic parameters (storage modulus G′ and loss modulus G′′) of the hydrogel were increased with the gradual increase of the GA fibril concentration (1–4 wt%) (see Fig. S2B, ESI†). Based on the synergistic combination of gelation and emulsifying behaviors of GA fibrils (Fig. 1 and S2, ESI†), it can be reasonably expected that the GA fibril-stabilized emulsions could be transformed into the structured emulsion system (i.e. emulsion gel) when the GA fibril concentration is higher than its minimum gelling concentration (around 0.5 wt%).Fig. 2Aa shows the effect of GA fibril concentrations (0.5–4 wt%) on the formation of emulsion gels, and the olive oil concentration was constant at 60 wt%. As can be seen, at 0.5% GA fibril concentration, the prepared emulsions were fluid in nature and did not show any gelation, although the fibrillar hydrogel can be formed at an identical fibril concentration (0.5% GA, Fig. 2Aa). Upon the further increase of fibril concentrations (1–4%), as expected, all these emulsions stabilized by GA fibril were able to be transformed into self-standing gels. The results indicate that through a facile one-pot emulsification at high temperature (80 °C) followed by a subsequent cooling procedure, the GA fibril-stabilized emulsion gels can be successfully created. The cooling could strengthen the noncovalent interactions among fibrils in the continuous phase as well as at the surface of emulsion droplets, leading to the formation of a fibrillar network structure, and then the construction of the soft solid emulsion gels was achieved. Furthermore, the impact of an oil phase fraction (10–60 wt%) on the formation of 4% GA fibril-based emulsion gels is shown in Fig. 2Ab. It can be clearly seen that, at lower oil fractions (10–20%), the emulsion gels showed an evident phase separation, where the upper phase was a porous emulsion gel but the bottom phase appeared to be the transparent GA hydrogel (0%, see Fig. 2Ab). On the basis of the assembly behavior of GA fibrils to form a hydrogel, we conclude that, at relatively lower oil concentrations (10–20%), there may be a large number of GA fibrils left in the continuous phase after their complete adsorption onto the oil droplet surface. Upon cooling, some of these free GA fibrils probably self-assemble into the hydrogel via interfibrillar interactions prior to the organization of emulsion gels,28,33 thus leading to the phase separation. Accordingly, with increasing oil phase concentrations (30–60%), a series of fine emulsion gels with a homogeneous appearance were obtained (Fig. 2Ab). From Fig. S3 (ESI†), it can be seen that these emulsion gels (60% oil) had a spread-like appearance and were easily spreadable, suggesting their potential applications in foods, cosmetics and pharmaceuticals, such as in spreads and ointments.
3.3 Microstructure of emulsion gels
3.4 Rheological behaviors
As can be seen, for all investigated cases, the elastic modulus (G′) was always significantly higher than the viscous modulus (G′′) in their individual linear viscoelastic regions (LVR), suggesting that the emulsion gels exhibit mostly an elastic solid-like behavior. With increasing amplitude, a distinct crossover or yield point (G′′ > G′) was found, indicating the yielding of the network structure at a higher stress amplitude. It can be seen from Fig. 5A that, with increasing GA fibril concentration, the emulsion gels displayed a relatively broader LVR, higher critical stress, and higher G′ values over the applied amplitude range, suggesting an increase in the gel strength. From Fig. 5B, it can be seen that the increase in the olive oil concentration also led to a progressive increase in the gel strength. As mentioned earlier, the GA fibril-coated droplets within the gel network may function as active filler particles, and thus their closing packing and connection with the gel matrix (see Fig. 2B and 4A) can contribute to the strengthening of the network structure.21,42 Note that, compared with the GA fibrillar hydrogel (Fig. S2, ESI†), the emulsion gels showed much higher G′ values, further confirming the role of the dispersed emulsion droplets in the gel network. These results are in agreement with the analyses of PLM (Fig. 2B) and CLSM (Fig. 4).
To investigate the dependence of the rheological response on frequency, frequency sweeps were performed. The frequency was increased from 0.1 to 100 rad s−1 at a constant stress of 1 Pa (within the LVR). As seen from Fig. 5C and D, both G′ and G′′ (especially the G′) for all the systems exhibited a relatively weak frequency dependence, and the G′ curves had slightly positive slopes, suggesting that the rheological response of emulsion gels is not obviously affected by the applied deformation rate.43,44 The loss tangent (G′′/G′) values were also low (below 0.1), except for the 1% GA fibril-stabilized emulsion gel (around 0.2–0.4). The results, together with the always much higher G′ values than the corresponding G′′ values over the entire frequency range, indicate that these emulsion gels have reasonably high gel strength, especially at higher GA fibril and olive oil concentrations. Moreover, the absence of a yield point (G′ = G′′) even at high frequency indicates that the emulsion gels do not have a gel–sol transformation even at a higher rate of deformation. Also, the G′ values significantly increased throughout the frequency range for emulsion gels with increasing concentrations of GA fibril (Fig. 5C) and olive oil (Fig. 5D). These results are in good agreement with the results of amplitude sweeps (Fig. 5A and B), suggesting that the emulsion gels with controlled strength and rheological properties can be engineered by simply varying the concentrations of GA fibril and the oil phase.
σ = σy + K![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) n n |
![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) = shear rate (s−1), and n = flow behavior index. The value of σy quantifies the amount of stress that a material withstands before it yields and begins to flow, and K is a measure of the consistency of the material.43,45 The value of n can be used to express the non-linearity of the stress–shear-rate relationship, and to define the degree of shear shinning (0 < n < 1) or shear thickening (n > 1) of the material.43,45 The calculated flow parameters including yield stress (σy), consistency coefficient (K), and flow behavior index (n) are listed in the inset table of Fig. 6A. As can be seen, the values of σy and K obviously increased with increasing GA fibril concentration, which is indicative of a more complex, denser and stronger network structure.46 The values of n of all the emulsion gels were smaller than unity, which confirms their shear thinning behavior. However, the degree of shear thinning was higher (lower n values) for the emulsion gels at higher GA fibril concentrations (3–4%), suggesting their higher non-linearity and hence stronger shear sensitivity. The shear thinning behavior of emulsion gels could be a consequence of the disruption of oil droplet clusters and the subsequent arrangement of these clusters in the flow direction.43,45 These results are consistent with the data from microstructure (Fig. 2B and 4A) and oscillatory measurements (Fig. 5A and C).
= shear rate (s−1), and n = flow behavior index. The value of σy quantifies the amount of stress that a material withstands before it yields and begins to flow, and K is a measure of the consistency of the material.43,45 The value of n can be used to express the non-linearity of the stress–shear-rate relationship, and to define the degree of shear shinning (0 < n < 1) or shear thickening (n > 1) of the material.43,45 The calculated flow parameters including yield stress (σy), consistency coefficient (K), and flow behavior index (n) are listed in the inset table of Fig. 6A. As can be seen, the values of σy and K obviously increased with increasing GA fibril concentration, which is indicative of a more complex, denser and stronger network structure.46 The values of n of all the emulsion gels were smaller than unity, which confirms their shear thinning behavior. However, the degree of shear thinning was higher (lower n values) for the emulsion gels at higher GA fibril concentrations (3–4%), suggesting their higher non-linearity and hence stronger shear sensitivity. The shear thinning behavior of emulsion gels could be a consequence of the disruption of oil droplet clusters and the subsequent arrangement of these clusters in the flow direction.43,45 These results are consistent with the data from microstructure (Fig. 2B and 4A) and oscillatory measurements (Fig. 5A and C).
The thixotropic behavior of the GA fibril-based emulsion gels was further studied to gain insights into their structure-recovery properties. The emulsion gel samples were subjected to three-interval time tests, wherein the viscosity of samples was monitored as a function of time under an alternate cycle of low and high shear rates (0.1, 10, and 0.1 s−1). As seen from Fig. 6B, at low GA fibril concentrations (1–2%), the viscosity of emulsion gels slightly increased with time at a low shear rate (0.1 s−1), showing a slight rheopexy behavior, which should be due to the clustering of oil droplets.45 In contrast, for the emulsion gels stabilized by high GA fibril concentrations (3–4%), there was no apparent change in the viscosity values over time at a low shear rate (0.1 s−1) in the first interval. When the shear rate was increased from 0.1 to 10 s−1 in the second interval, the shear sensitivity of all emulsion gels became evident with a drop in the viscosity values. However, the emulsion gels (especially at 3–4% GA fibril) showed an almost complete recovery when the shear rate was changed back to 0.1 s−1. We further quantitatively analyzed the structure recovery degree of emulsion gels by taking the viscosity value at the end of the first interval as reference (100%) and comparing it with the maximum viscosity value in the third interval. The recovery percentage for all emulsion gels was in the range of 60–90%, and the percentage increased with increasing GA fibril concentration, which indicates that the GA fibril-stabilized emulsion gels (especially at 3–4% GA fibril concentration) have a good structure recovery at rest. This can be explained by assuming that the clusters of closely packed droplets, which were oriented in the direction of flow at a high shear rate (10 s−1), may return back to a more random distribution at a low shear rate (0.1 s−1), thus resulting in an increase in the dissipated energy or resistance to flow.44,46
3.5 Large deformation mechanical properties
The large deformation compression study was carried out to further understand the impact of GA fibril concentrations on the structure of emulsion gels. Fig. S4 (ESI†) shows the force–relative displacement curves and yield force (hardness) of emulsion gels as a function of GA fibril concentrations (1–4%). It can be seen that, as expected, the increase in GA fibril concentration had a great effect on the enhancement of mechanical properties of emulsion gels. At low GA fibril concentrations (1%), the emulsion gel displayed a very weak response curve and a low hardness value (0.51 N), suggesting a weak gel structure. With increasing GA fibril concentration, the response of the curve and the hardness value increased significantly. Particularly at 4% GA fibril concentration, the emulsion gel showed an apparent strain-hardening behavior upon compression, with a relatively high hardness value (6.94 N), indicating a stronger gel network. The results are in good agreement with the results obtained from the microstructure observations (Fig. 2B and 4) and the small deformation rheological measurements (Fig. 5 and 6) of these emulsion gels.3.6 Thermoresponsive properties of emulsion gels
Owing to the noncovalent cross-links between fibrils, the GA fibrillar hydrogel is known to be thermoreversible with a gel–sol transition temperature range of 55–60 °C, and above this temperature range, the fibrillar network initiates melting.33 Therefore, due to the fibrillar network structure, the GA fibril-stabilized emulsion gels should have an interesting responsive behavior as a function of temperature. As expected, it can be seen that all the emulsion gels (1–4% GA fibrils) showed a clear gel-to-sol transition during heating and a reversible gelation during cooling (Fig. 7A, B, S5A, and S5B, ESI†). The G′ and G′′ values measured during heating and cooling cycles suggest a good thermo-reversibility of emulsion gels. We further used PLM to observe the changes in the microstructure of emulsion gels stabilized by 4% GA fibril during heating and cooling cycles (Fig. S5C, ESI†). As can be seen, when the samples were heated from 25 to 80 °C, the light for the fibrillar network in the continuous phase disappeared gradually, and during cooling, it emerged again, suggesting the reversibility of thermal transition (Fig. 7B). However, the emulsion droplets within the gel network remained stable throughout the studied temperature (see Fig. S5C, ESI†), which could be explained by the multilamellar GA fibril shells with high electrostatic forces (Fig. 1 and 3). The results suggest that the GA fibril-stabilized emulsion gels also have the thermoreversible behavior, which is mainly attributed to the noncovalent fibril network in the continuous phase.28,29,33 Thus, by simply changing the temperature, the emulsion gels can be switched reversibly between a gel and liquid dispersion (sol).The temperature-responsive behavior of the GA fibril-stabilized emulsion gels was further utilized to obtain the soft solid oil products with predetermined shapes by simply extruding the warm emulsion gels (around 50–60 °C, weak gel or sol) using a plastic syringe. Fig. 7C shows the photographs of the emulsion gels with the shape of alphabets after cooling the extruded warm emulsion gels (2 and 4% GA fibrils) at room temperature (25 °C) for 1 min. In this way, we demonstrated a simple exploitation of the thermoresponsive GA fibril-based emulsion gels to prepare soft structures with controlled shapes.
3.7 Stability and delivery vehicle for functionality
In this section, we further evaluated the stability of the emulsion gels during storage. The stored samples were periodically determined using oscillatory frequency sweeps (0.1–100 rad s−1, stress = 1 Pa) to monitor the structural changes over 30 days of storage at room temperature (25 °C). Fig. S6A (ESI†) shows the rheological properties and appearance of the emulsion gels as a function of time, and the results confirmed the excellent stability of these emulsion gels (2–4% GA fibrils), except for the 1% GA fibril-stabilized gel sample, which showed a decrease in the G′ values after 30 days. The emulsion gel (4% GA fibril) was then subjected to water removal through lyophilization. As can be seen from Fig. S6B (ESI†), a white gel-like solid was formed without any oil leakage, indicating that the oil droplets covered by GA fibril shells could maintain good stability during freeze-drying. This result suggests that the GA fibril-based emulsion gels could be a potential template for producing gel-like oil products or the so-called oil gels, which contained nearly 94 wt% liquid oil. On the basis of the earlier analysis (Fig. 1–3 and 7) it is seen that the observed stability of emulsion gels during storage and drying is mainly due to the multilayer fibril shells of emulsion droplets with high electrostatic repulsive forces, which provide high electrostatic stability for the GA fibril-stabilized emulsion gels. Furthermore, the close packing of emulsion droplets within the continuous network also contributes to the strengthening of the gel network structure thus increasing the stability of emulsion gels (Fig. 2B and 4). Another important application for emulsion gels is their use as delivery systems for oil-soluble functional ingredients in foods, pharmaceuticals, and cosmetics. Herein, we further fabricated the functional emulsion gels stabilized by GA fibrils by simply dissolving oil-soluble β-carotene (model compound) into the oil prior to the homogenization step, to evaluate the loading capacity of oil-soluble bioactives into emulsion gels. From Fig. S6C (ESI†), it can be seen that the addition of β-carotene did not affect the formation and stabilization of emulsion gels (1–4% GA fibrils). In addition, these functional emulsion gels also showed excellent stability without showing any significant changes in the appearance after 30 days, which is confirmed by the frequency sweep result (Fig. S6C, ESI†). These results indicate that the GA fibril-based emulsion gels could be used for delivery purposes in foods, cosmetics, and pharmaceutical applications.4. Conclusions
In this work, we showed that the natural saponin GA could be used as a novel material to structure a vegetable oil into the soft solid emulsion gels. The GA nanofibrils from the supramolecular self-assembly of GA molecules were first used as stabilizers to fabricate O/W emulsions by a simple one-step emulsification at high temperature (80 °C), and then a subsequent cooling was applied to trigger the formation of the GA fibril-based emulsion gels with a high oil fraction. The cooling could strengthen the noncovalent interactions between GA fibrils in the continuous phase as well as at the droplet surface, leading to the formation of an assembled fibrillar network structure. We found that the GA fibrils had a multilayer adsorption at the droplet surface, yielding a multilayer interfacial fibril structure. Such unique multilayer fibril shells of droplets with high electrostatic force could provide superior stability for the GA fibril-stabilized emulsion droplets and thus the whole emulsion gel during storage and heating. The thermoreversible gel–sol transitions of the self-assembled GA fibrillar network in the continuous phase led to the temperature-responsive behavior of emulsion gels. In addition, the emulsion gels showed many encouraging rheological properties, and have the potential to be utilized as stable delivery systems for functional ingredients. To our knowledge, this is the first study where the natural triterpenoid saponins and their interesting self-assembly behaviors are used in designing and constructing temperature-responsive vegetable oil soft solid products. We expect that these simple and inexpensive, smart, responsive, oil structuring based natural saponins could find a broad range of applications in e.g. foods, pharmaceuticals, and cosmetics.Acknowledgements
Financial support by the General Project of China Postdoctoral Science Foundation and the National Natural Science Foundation of China (serial numbers 31371744 and 31501425) is kindly acknowledged.References
- A. Vintiloiu and J. C. Leroux, J. Controlled Release, 2008, 125, 179–192 CrossRef CAS PubMed.
- L. Chen, G. E. Remondetto and M. Subirade, Trends Food Sci. Technol., 2006, 17, 272–283 CrossRef CAS.
- N. E. Hughes, A. G. Marangoni, A. J. Wright, M. A. Rogers and J. W. Rush, Trends Food Sci. Technol., 2009, 20, 470–480 CrossRef CAS.
- M. Pernetti, K. F. van Malssen, E. Flöter and A. Bot, Curr. Opin. Colloid Interface Sci., 2007, 12, 221–231 CrossRef CAS.
- A. G. Marangoni, J. Am. Oil Chem. Soc., 2012, 89, 749–780 CrossRef CAS.
- S. Simovic, J. Milic-Askrabic, G. Vuleta, S. Ibric and M. Stupar, Int. J. Cosmet. Sci., 1999, 21, 119–125 CAS.
- J. M. Aiache, P. Gauthier and S. Aiache, Int. J. Cosmet. Sci., 1992, 14, 228–234 CrossRef CAS PubMed.
- R. Sánchez, J. M. Franco, M. A. Delgado, C. Valencia and C. Gallegos, Green Chem., 2009, 11, 686–693 RSC.
- F. C. Wang, A. J. Gravelle, A. I. Blake and A. G. Marangoni, Curr. Opin. Food Sci., 2016, 7, 27–34 CrossRef.
- M. A. Rogers, A. J. Wright and A. G. Marangoni, Curr. Opin. Colloid Interface Sci., 2009, 14, 33–42 CrossRef CAS.
- A. J. Wright and A. G. Marangoni, J. Am. Oil Chem. Soc., 2006, 83, 497–503 CrossRef CAS.
- H. S. Hwang, S. Kim, M. Singh, J. K. Winkler-Moser and S. X. Liu, J. Am. Oil Chem. Soc., 2012, 89, 639–647 CrossRef CAS.
- A. R. Patel, M. Babaahmadi, A. Lesaffer and K. Dewettinck, J. Agric. Food Chem., 2015, 63, 4862–4869 CrossRef CAS PubMed.
- H. Schaink, K. Van Malssen, S. Morgado-Alves, D. Kalnin and E. van der Linden, Food Res. Int., 2007, 40, 1185–1193 CrossRef CAS.
- C. V. Nikiforidis and E. Scholten, RSC Adv., 2014, 4, 2466–2473 RSC.
- A. Bot and W. G. Agterof, J. Am. Oil Chem. Soc., 2006, 83, 513–521 CrossRef CAS.
- T. Laredo, S. Barbut and A. G. Marangoni, Soft Matter, 2011, 7, 2734–2743 RSC.
- M. Davidovich-Pinhas, S. Barbut and A. Marangoni, Carbohydr. Polym., 2015, 117, 869–878 CrossRef CAS PubMed.
- Y. Huang, M. He, A. Lu, W. Zhou, S. D. Stoyanov, E. G. Pelan and L. Zhang, Langmuir, 2015, 31, 1641–1648 CrossRef CAS PubMed.
- C. V. Nikiforidis and E. Scholten, RSC Adv., 2015, 5, 37789–37799 RSC.
- E. Dickinson, Food Hydrocolloids, 2012, 28, 224–241 CrossRef CAS.
- C. Rodríguez-Abreu and M. Lazzari, Curr. Opin. Colloid Interface Sci., 2008, 13, 198–205 CrossRef.
- H. D. Batte, A. J. Wright, J. W. Rush, S. H. Idziak and A. G. Marangoni, Food Biophys., 2007, 2, 29–37 CrossRef.
- J. Lu, J. Hu, Y. Song and Y. Ju, Org. Lett., 2011, 13, 3372–3375 CrossRef CAS PubMed.
- J. Hu, M. Zhang and Y. Ju, Soft Matter, 2009, 5, 4971–4974 RSC.
- B. G. Bag, S. K. Dinda, P. P. Dey, V. A. Mallia and R. G. Weiss, Langmuir, 2009, 25, 8663–8671 CrossRef CAS PubMed.
- B. G. Bag and R. Majumdar, RSC Adv., 2012, 2, 8623–8626 RSC.
- A. Saha, J. Adamcik, S. Bolisetty, S. Handschin and R. Mezzenga, Angew. Chem., Int. Ed., 2015, 127, 5498–5502 CrossRef.
- L. A. Estroff and A. D. Hamilton, Chem. Rev., 2004, 104, 1201–1217 CrossRef CAS PubMed.
- Y. Gao, Y. Li, X. Zhao, J. Hu and Y. Ju, RSC Adv., 2015, 5, 102097–102100 RSC.
- J. Lu, Y. Gao, J. Wu and Y. Ju, RSC Adv., 2013, 3, 23548–23552 RSC.
- M. N. Asl and H. Hosseinzadeh, Phytother. Res., 2008, 22, 709–724 CrossRef CAS PubMed.
- H. Yoshioka, J. Colloid Interface Sci., 1985, 105, 65–72 CrossRef CAS.
- R. A. Carlton, Polarized light microscopy, in Pharmaceutical microscopy, ed. R. A. Carlton, Springer, 2011, pp. 7–64 Search PubMed.
- N.-P. K. Humblet-Hua, E. van der Linden and L. M. Sagis, Soft Matter, 2013, 9, 2154–2165 RSC.
- Z. Wan, X. Yang and L. M. Sagis, Langmuir, 2016, 32, 3679–3690 CrossRef CAS PubMed.
- M. A. Bos and T. van Vliet, Adv. Colloid Interface Sci., 2001, 91, 437–471 CrossRef CAS PubMed.
- K. Golemanov, S. Tcholakova, N. Denkov, E. Pelan and S. D. Stoyanov, Soft Matter, 2013, 9, 5738–5752 RSC.
- A. I. Romoscanu and R. Mezzenga, Langmuir, 2006, 22, 7812–7818 CrossRef CAS PubMed.
- N. R. Cameron and D. C. Sherrington, Adv. Polym. Sci., 1996, 126, 163–214 CrossRef CAS.
- R. Khurana, C. Coleman, C. Ionescu-Zanetti, S. A. Carter, V. Krishna, R. K. Grover, R. Roy and S. Singh, J. Struct. Biol., 2005, 151, 229–238 CrossRef CAS PubMed.
- E. Dickinson and J. Chen, J. Dispersion Sci. Technol., 1999, 20, 197–213 CrossRef CAS.
- J. W. Goodwin and R. W. Hughes, Rheology for chemists: an introduction, Royal Society of Chemistry, Cambridge, 2008 Search PubMed.
- C. Tropea, A. L. Yarin and J. F. Foss, Springer handbook of experimental fluid mechanics, Springer Science & Business Media, Berlin, 2007 Search PubMed.
- A. P. Deshpande, J. M. Krishnan and S. Kumar, Rheology of complex fluids, Springer Science & Business Media, New York, 2010 Search PubMed.
- T. G. Mezger, The rheology handbook: for users of rotational and oscillatory rheometers, Vincentz Network GmbH & Co. KG, Hannover, 2006 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6fo01485b |
| This journal is © The Royal Society of Chemistry 2017 |