Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of hydraulic retention time and substrate availability in denitrifying bioelectrochemical systems
Narcis
Pous
 ,
Sebastià
Puig
,
Sebastià
Puig
 *,
M. Dolors
Balaguer
*,
M. Dolors
Balaguer
 and
Jesús
Colprim
and
Jesús
Colprim

Laboratory of Chemical and Environmental Engineering (LEQUiA), Institute of the Environment, University of Girona, Campus Montilivi, Carrer Maria Aurèlia Capmany, 69, E-17003 Girona, Spain. E-mail: sebastia@lequia.udg.cat; Fax: +34 972418150; Tel: +34 972418182
First published on 29th June 2017
Abstract
Denitrifying bioelectrochemical systems (BES) allow safe nitrate treatment in waters with low organic carbon content without chemical requirements and at a competitive cost. However, this technology should move towards scaling-up by improving removal rate capabilities. In this study, a novel tubular design was used to evaluate whether the hydraulic retention time and the influent nitrate concentration influence the nitrate removal rate of denitrifying BES. A nitrate consumption rate of up to 849 g N mNCC−3 d−1 was reached without accumulation of nitrites at a HRT of 28 minutes. Nitrate removal activity was evaluated under different nitrate influent concentrations and under different HRTs. Results suggested preeminence of HRT on modulating the denitrifying activity. Therefore, this study presents an innovative design for nitrate removal using denitrifying BES and it demonstrates that operation at low HRTs increases the nitrate removal rate. It suggests that an appropriate approximation of scaling-up denitrifying BES would be the implementation of compact reactors connected in series operated at low HRTs.
Water impactBioelectrochemical systems are a promising alternative for dealing with nitrates in waters with low organic matter content. Their future implementation depends on achieving higher treatment performances. We demonstrated that high nitrate removal rates can be obtained by decreasing the HRT. Operation at low HRTs requires lower reactor volumes, which increases the denitrifying BES market opportunities. |
1. Introduction
The presence of nitrate in groundwater, surface water and wastewater demands the investigation of innovative technologies for its removal.1 Bioelectrochemical systems (BES) could emerge as an alternative technology for nitrate treatment.2,3 Market opportunities can be found in different kinds of waters that present a lack of organic matter content: i) urban wastewaters with a low C/N ratio, where denitrifying BES could be applied as a tertiary treatment for nitrogen polishing;4 ii) the anammox process, according to stoichiometry, releases 16.1% of initial ammonium as nitrate, thus the anammox effluent might require a nitrate post-treatment;5 iii) nitrate accumulates in aquaculture systems which harms fish production,6 thus requiring nitrate treatment with external electron donor supply;7 iv) nitrate-polluted groundwater is a worldwide concern.8–10BES are usually based on an anode and a cathode separated by an ion exchange membrane.11 When a BES aims to treat nitrate, the cathode is colonized by autotrophic denitrifying bacteria. They are able to reduce nitrate to nitrogen gas using an electrode as an electron donor.12–15 Different reactions can be used at the anode compartment. Anodic organic matter oxidation is the most common configuration.2,3 However, this operational strategy applied to waters with low organic matter content would require chemical addition (i.e. acetate enriched solution). In previous studies, it has been demonstrated that water (instead of organic matter) can be successfully used as an anode electron donor if external energy is applied (either by supplying a constant current16,17 or by controlling the cathode potential18,19). In consequence, BES become a sustainable technology to treat nitrates: i) no organic matter/chemical addition is required; ii) low energy consumption is needed (0.68 × 10−2 kW h g N-NO3−removed; 0.20 kW h mtreated−3)19 with respect to competing technologies for nitrate removal (membrane bioreactors or biofilm-electrode reactors (2.04 × 10−2 and 7.00 × 10−2 kW h g N-NO3−removed, respectively))17,20 or competing technologies for nitrate separation (electrodialysis or reverse osmosis (0.69 and 1.03 kW h mtreated−3, respectively)).21 However, considering the high capital costs required for implementing BES-based technologies,22,23 nitrate reduction rates should outperform current treatments. To date, the highest nitrate removal rate in denitrifying BES has been described by Clauwaert et al. (2009) using a microbial fuel cell (MFC) with pH control for treating synthetic wastewater (503 g N m−3 d−1).24 This value is below those of other organic carbon-free technologies for treating nitrates, such as hydrogenotrophic denitrification (up to 770 g N m−3 d−1 treating nitrate-polluted groundwater).25
One of the parameters that might be limiting the current BES performances is an improper water distribution inside the reactor.26 A non-appropriate hydrodynamics can lead to heterogeneous colonization of the cathode compartment.18 As a result, zones with different denitrification potentials can appear.18 In order to improve the water flux distribution inside the reactor, efforts can be made on improving the reactor design and operation.26 For these reasons, this work evaluates the application of high influent flow-rates in a novel tubular denitrifying BES. We investigated the effect of the hydraulic retention time (HRT) and nitrate influent concentration on nitrate removal rates.
2. Methods
2.1. Experimental set-up
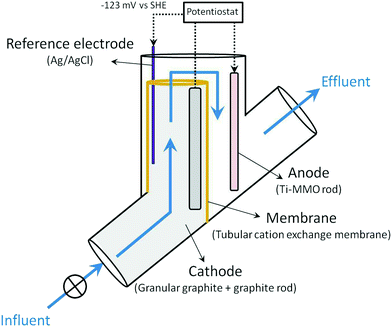
Two identical denitrifying BES reactors were assembled for performing the experiments (replicates 1 and 2). Fig. 1 shows the schematic representation of the reactors. The denitrifying BES consisted of a tubular reactor where the two compartments (anode and cathode) were separated by a tubular cation exchange membrane (CEM, CMI-7000, Membranes Int., USA). The cathode was located at the inner part of the reactor, and the anode in the outer. The cathode was filled with granular graphite (diameter 1.5–5 mm, EnViro-cell, Germany) and a graphite rod (250 × 6 mm, Mersen Ibérica, Spain) used as the electrode collector. The resulting net cathode volume (net cathode compartment volume – NCC) was 0.24 L.A Ti-MMO electrode rod (225 × 6 mm, NMT electrodes, South Africa) was used as the anode electrode. Ti-MMO electrodes are able to promote chloride oxidation to chlorine.27 However, no chlorine production was observed in the denitrifying BES of this study.
An Ag/AgCl reference electrode (+0.197 V vs. standard hydrogen electrode (SHE), model RE-5B BASi, USA) was introduced in the cathode compartment. The cathode potential was poised at −0.320 V vs. Ag/AgCl using a potentiostat (VSP, Bio-logic, France) according to previous knowledge.19
A flow-through configuration was used to reduce the number of pumps needed. Nitrate-contaminated water was directly fed to the bottom of the cathode compartment (inner part of the reactor), and spilled from the top to the anode compartment (outer part of the reactor). The system was thermostatically controlled at 22 ± 1 °C.
2.2. BES inoculation and operation
In both replicates, the cathode was inoculated with the effluent of a parent denitrifying BES and operated under fed-batch mode during the first 10 days.19 During the inoculation, the BES was connected in closed-loop mode to a 2.5 L tank. The tank was filled with 1.5 L of the influent medium (described in section 2.3.) with 1.0 L of the effluent of a parent denitrifying BES. The medium was replaced with new fresh medium when nitrate was consumed to below 1 mg N-NO3− L−1. Then, the denitrifying BES was fed under continuous-flow mode at a flow of 0.6 and 0.5 L d−1, corresponding to a cathodic HRT of 9.60 h and 10.89 h in replicate 1 and 2, respectively. After eight days of operation, the current and the nitrate removal rate stabilized (steady-state conditions were reached). Then, the different hydraulic retention times were tested.2.3. Experimental procedure to evaluate the effect of the hydraulic retention time and the nitrate influent concentration on nitrate removal performance
The denitrifying BES were fed with an organic-carbon-free synthetic medium prepared with distilled water. It contained 0.20 g L−1 NaNO3 (33 mg N-NO3− L−1); 1.05 g L−1 NaHCO3 as the inorganic carbon source; 0.32 g L−1 Na2HPO4·7H2O; 2.14 g L−1 KH2PO4·H2O; 0.50 g L−1 NaCl; 0.10 g L−1 MgSO4·7H2O; 0.02 g L−1 CaCl2; 0.02 g L−1 NH4Cl; and 0.1 mL L−1 trace nutrients.28 Media were flushed with N2 gas for 15 minutes prior to feeding. Different HRTs from the initial 10.89 to 0.46 h (28 min) were applied. Every HRT was maintained for seven days. Five samples were taken and analyzed at every test (one per day, from the 3rd day to the 7th).The nitrate removal activity was also evaluated at different nitrate influent concentrations. In order to evaluate the effect of different nitrate contents on nitrate removal performance, the influent medium was spiked with different nitrate concentrations from 0.04 to 0.68 g L−1 NaNO3 (from 6.5 to 112.0 mg N-NO3− L−1). The conductivity of the medium was adjusted to 4.1 mS cm−1 by controlling the Na+ content at 0.25 g L−1 Na+ through spiking different NaCl concentrations from 0.61 to 0.17 g L−1 NaCl. The conductivity was set constant in all tests to avoid its influence on bioelectrochemical denitrification.8 The set of different nitrate influent contents was evaluated at three different HRTs: 1.2, 1.6 and 3.4 h.
2.4. Analytical methods and calculations
Samples from the effluent of the reactor were regularly taken and analyzed. Standard wastewater measurements of ammonium (N-NH4+), nitrites (N-NO2−) and nitrates (N-NO3−) were taken and analyzed according to the recommendations of the American Public Health Association (APHA).29 The concentration of N2O was measured with a N2O liquid-phase microsensor (Unisense, Denmark) located at the BES effluent. Free chlorine was analyzed using photometric kits (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 595 chlorine cell tests Spectroquant®, Merck, Germany). The pH and conductivity were measured with a pH-meter (pH-meter basic 20+, Crison, Spain) and an EC-meter (EC-meter basic 30+, Crison, Spain), respectively.
595 chlorine cell tests Spectroquant®, Merck, Germany). The pH and conductivity were measured with a pH-meter (pH-meter basic 20+, Crison, Spain) and an EC-meter (EC-meter basic 30+, Crison, Spain), respectively.
In order to know the performance of each reduction step from NO3− to N2, the rates of nitrate, nitrite and nitrous oxide reduction (rNO3−, rNO2− and rN2O, respectively) were calculated (eqn (1) to (3)). Nitric oxide accumulation was considered negligible.3
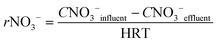 | (1) |
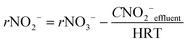 | (2) |
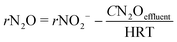 | (3) |
The coulombic efficiency (CE) of the denitrifying biocathode was calculated by adapting the equation proposed by Virdis et al. (2009) but taking into account the required current for each sequential step of nitrate reduction to dinitrogen gas.3,18 The reduction steps calculated were: the nitrate reduction to nitrite (NO3−/NO2−), nitrite to nitrous oxide (it includes NO reduction to N2O; NO2−/N2O) and nitrous oxide to dinitrogen gas (N2O/N2). The CE was calculated as shown in eqn (4).
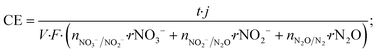 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1 e−); n accounts for the number of electrons required for each reaction (nNO3−/NO2− = 2, nNO2−/N2O = 2 and nN2O/N2 = 1); rNO3−, rNO2− and rN2O are the consumption rates calculated in mmol N LNCC−1 h−1.
485 C mol−1 e−); n accounts for the number of electrons required for each reaction (nNO3−/NO2− = 2, nNO2−/N2O = 2 and nN2O/N2 = 1); rNO3−, rNO2− and rN2O are the consumption rates calculated in mmol N LNCC−1 h−1.
3. Results and discussion
3.1. Denitrification performance in a tubular denitrifying BES operated at low HRTs
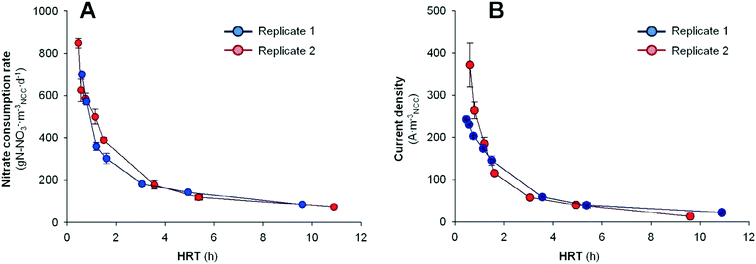
After the inoculation period (10 days), both denitrifying BES were fed in continuous-flow mode at a HRT of 9.60 h and 10.89 h and operated at a poised cathode potential of −0.320 V vs. Ag/AgCl.19 Eight days later, the denitrifying BES reached stable behavior with a current density of 13.8 ± 1.3 A mNCC−3 and 100% nitrate removal efficiency in replicate 1, and 22.4 ± 0.7 A mNCC−3 and 94% in replicate 2. It implied a nitrate consumption rate of 85 ± 0 g N mNCC−3 d−1 and 73 ± 5 g N mNCC−3 d−1, respectively. Moreover, nitrite was not accumulated. Hence, the denitrifying BES allowed a complete removal of nitrate without nitrite accumulation.In order to maximize the nitrate consumption rate in the tubular denitrifying BES, the nitrogen loading rate was increased by decreasing the HRT from the initial 10.89 to 0.46 h. Fig. 2 shows the nitrate removal rate (rNO3−) according to the different applied HRTs. The whole set of data is reported in Table 1.
| BES | HRT (h) | NO3− consumption rate (g N-NO3− mNCC−3 d−1) | Nitrogen content | |||
|---|---|---|---|---|---|---|
| NO3− influent (mg N-NO3− L−1) | NO3− effluent (mg N-NO3− L−1) | NO2− effluent (mg N-NO2− L−1) | N2O effluent (mg N-N2O L−1) | |||
| Replicate 1 | 9.60 ± 0.00 | 85 ± 0 | 33.8 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | — |
| 4.93 ± 0.06 | 144 ± 3 | 33.8 ± 0.0 | 4.3 ± 0.3 | 0.0 ± 0.0 | — | |
| 3.06 ± 0.00 | 182 ± 10 | 33.8 ± 0.3 | 10.6 ± 1.4 | 0.0 ± 0.0 | — | |
| 1.61 ± 0.02 | 302 ± 26 | 32.8 ± 1.1 | 12.6 ± 1.3 | 0.0 ± 0.0 | 11.2 ± 0.0 | |
| 1.19 ± 0.02 | 359 ± 19 | 31.3 ± 2.1 | 13.4 ± 1.3 | 0.0 ± 0.0 | 13.7 ± 1.2 | |
| 0.79 ± 0.01 | 273 ± 15 | 33.4 ± 0.8 | 14.6 ± 0.8 | 0.0 ± 0.0 | 8.6 ± 0.9 | |
| 0.61 ± 0.00 | 700 ± 7 | 32.5 ± 0.1 | 14.9 ± 0.2 | 0.0 ± 0.0 | 5.9 ± 1.7 | |
| Replicate 2 | 10.89 ± 0.00 | 73 ± 5 | 35.3 ± 1.4 | 2.1 ± 2.7 | 0.0 ± 0.0 | 15.3 ± 0.4 |
| 5.37 ± 0.15 | 119 ± 13 | 34.2 ± 2.4 | 7.5 ± 1.3 | 0.0 ± 0.0 | 21.1 ± 3.1 | |
| 3.56 ± 0.08 | 178 ± 20 | 32.7 ± 1.0 | 6.4 ± 2.9 | 0.0 ± 0.0 | 23.5 ± 3.2 | |
| 1.49 ± 0.01 | 389 ± 13 | 30.5 ± 1.3 | 6.3 ± 2.1 | 0.0 ± 0.0 | 21.7 ± 1.1 | |
| 1.15 ± 0.03 | 500 ± 36 | 36.0 ± 1.7 | 12.1 ± 1.9 | 0.0 ± 0.0 | 20.6 ± 0.5 | |
| 0.75 ± 0.01 | 587 ± 25 | 32.7 ± 0.4 | 14.4 ± 0.3 | 0.0 ± 0.0 | 13.7 ± 0.9 | |
| 0.57 ± 0.00 | 626 ± 54 | 32.4 ± 0.1 | 17.5 ± 1.2 | 0.0 ± 0.0 | 8.5 ± 0.5 | |
| 0.46 ± 0.01 | 849 ± 23 | 32.7 ± 0.3 | 16.4 ± 0.2 | 0.0 ± 0.0 | 7.9 ± 0.8 | |
The nitrogen removal capabilities were clearly enhanced by decreasing the HRT. In replicate 1, the nitrate consumption rate and current demand increased from 85 ± 0 g N mNCC−3 d−1 (100% nitrate removed) and 13.8 ± 1.3 A mNCC−3 at 9.60 h to 700 ± 7 g N mNCC−3 d−1 (55% nitrate removed) and 371.5 ± 52.0 A mNCC−3 at 0.60 h. Meanwhile in replicate 2, by decreasing the HRT from 10.89 h to 0.46 h, the rNO3− increased from 73 ± 5 g N mNCC−3 d−1 (94% nitrate removed) and 22.4 ± 0.7 A mNCC−3 to 849 ± 23 g N mNCC−3 d−1 (50% nitrate removed) and 242.8 ± 1.7 A mNCC−3. Despite that the effluent nitrate concentrations were 16.4 ± 0.2 mg N L−1 (from an influent concentration of 32.7 ± 0.2 mg N L−1) when the system was removing nitrates at 849 ± 23 g N mNCC−3 d−1, the results presented here are relevant. For a general BES scale-up, the usage of small reactors connected in series has been seen as the most appropriate methodology.30 The capacity of removing nitrate at a fast rate when operating the system at 0.46 h implies that lower reactor volumes can be used for scaling-up denitrifying BES. Thus, a possible scaling-up of nitrate treatment in waters with low carbon content using BES could follow the strategy of operating different compact denitrifying BES in series.
If the CE values are considered, slight differences in replicate 1 and replicate 2 were observed. In replicate 1, the CE increased from 53 ± 5% at HRT 9.6 h to 127 ± 1% at HRT 0.6 h. Meanwhile, in replicate 2, the CE among the different tests ranged from 85 ± 7% at HRT 10.9 h to 123 ± 1% at HRT 0.4 h. At high HRTs, the slow water flow-rate may allow endogenous heterotrophic denitrifying bacteria to grow,31 allowing the removal of more nitrate than the observed current could sustain. As the flow-rate became faster (HRT is decreased), the denitrifying activity started to couple with the current demand, and CEs around 100% could be observed (between 4.9–1.6 h in replicate 1 and between 5.4–0.5 h in replicate 2). Finally, at high flow-rates (low HRTs), a surge of CE above 100% was detected, indicating an excess of current supply with respect to the observed denitrification rates. In terms of energy consumption, the two replicates differed due to the different CE trends observed. In replicate 1, at the highest performance (HRT of 0.60 h and rNO3− of 700 ± 7 g N mNCC−3 d−1), an energy consumption of 1.48 × 10−2 ± 0.03 × 10−2 kW h g N-NO3−removed (0.261 ± 0.003 kW h mtreated−3) was observed. Meanwhile in replicate 2, which presented a more stable CE trend, an energy consumption of 0.89 × 10−2 ± 0.02 × 10−2 kW h g N-NO3−removed (0.145 ± 0.003 kW h mtreated−3) was observed at the highest performance (HRT of 0.46 h and rNO3− of 849 ± 23 g N mNCC−3 d−1). These values are slightly higher than those values previously observed in our previous studies using a denitrifying BES operated in a three-electrode configuration (poised cathode potential of −0.320 V vs. Ag/AgCl) and treating water without organic matter content, but with a different reactor design (0.68 × 10−2 kW h g N-NO3−removed; 0.20 kW h mtreated−3).19 In that case, the reactor presented a rectangular shape and two pumps were used (influent and recirculation). While in the current work the reactor presented a tubular shape and only one pump has been used (influent). Moreover, the energy consumption observed here was still below the values observed in competing technologies for nitrate removal (membrane bioreactors or biofilm-electrode reactors (2.04 × 10−2 and 7.00 × 10−2 kW h g N-NO3−removed, respectively))17,20 or competing technologies for nitrate separation (electrodialysis or reverse osmosis (0.69 and 1.03 kW h mtreated−3, respectively)).21
To the best of the author's knowledge, the highest nitrate consumption rates reported in denitrifying biocathodes of BES were 483 and 503 g N mNCC−3 d−1, respectively.9,24 The operation proposed in this study was able to increase the reported values, increasing the possibilities of BES for nitrate removal. Not only were higher nitrate removal rates observed, but they were also achieved by operating the system at low HRTs (0.46–0.60 h). The operation at low HRTs improved the denitrifying activity in the denitrifying BES. The feasibility of getting high removal rates at low HRTs implies that the reactor size can be diminished. For a fixed influent water flow that has to be treated, a change in the HRT implies a change in the reactor volume. If the system can be operated at a low HRT, a lower reactor volume will be needed. This has a relevant impact on the application of denitrifying BES. In compact bioelectrochemical reactors, lower overpotentials are expected, and thus, higher treatment efficiencies can be reached.32 Moreover, the usage of compact reactors also implies a lower space demand (lower capital cost). For denitrifying BES application, the usage of compact reactors connected in series might be recommended to obtain both high nitrate removal rates and low effluent nitrate concentrations.
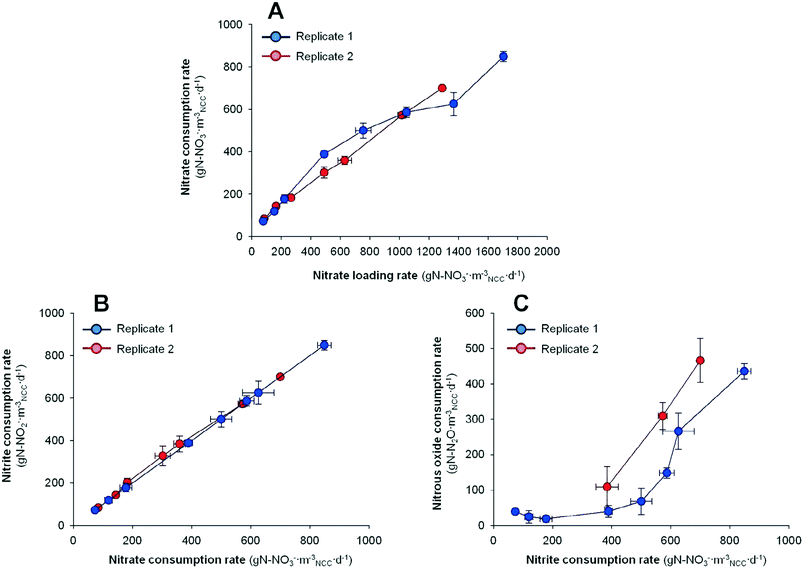
In order to consider the whole denitrifying pathway response, Fig. 3 shows the evolution of nitrite removal and nitrous oxide removal rates compared to the different substrate availabilities. The substrate availability (NO2− and N2O availability) resulted from the denitrifying activity at the different HRTs (rNO3− for NO2− availability and rNO2− for N2O availability). It can be observed that the decrease in the HRT (increase of nitrite loading rate) not only promoted the nitrate reduction, but also enhanced the reduction of denitrification intermediates.
No accumulation of nitrite was detected at any HRT neither at any replicate, suggesting that the nitrite reduction was faster than the nitrate removal step. On the contrary, nitrous oxide accumulation was detected. The nitrous oxide removal rate increased as the HRT was reduced. In replicate 1, the rN2O increased from 109 ± 57 to 466 ± 62 g N mNCC−3 d−1 by lowering the HRT from 1.19 to 0.60 h. In replicate 2, the rN2O increased from 39 ± 6 to 436 ± 21 g N mNCC−3 d−1 by lowering the HRT from 10.89 to 0.46 h. When the results are compared to N2O availability (thus compared with the nitrite removal rate) it highlights that the availability of N2O at higher HRTs did not result in a higher N2O removal rate, but it did at lower HRTs. Hence, the N2O biocatalysis in this BES was basically dominated by the hydrodynamics in the system rather than the substrate availability.
If the N2O results are weighted by the emissions of N2O (ratio between N2O and removed NO3−), a decrease with the HRT can be detected. In replicate 1, the emissions of N2O decreased from 77 to 33% by lowering the HRT from 1.19 to 0.60 h. In replicate 2, the N2O emission was 46% of the nitrate removed at 10.89 h. It increased up to 90% at 1.5 h and it finally decreased down to 48% at 0.46 h. Compared to previous studies in our group,18,19 as well as other authors applying potentiostatic conditions,3 the reported N2O emission values are relatively higher. The difference in N2O emissions could be explained through the different reactor design used. Virdis et al. (2009) and Pous et al. (2015a and 2015b) used a rectangular reactor operated with two pumps, influent flow and internal recirculation.3,18,19 While in the current work we performed the experiments in a tubular reactor without internal recirculation, only one pump was used for the whole system. Nevertheless, the N2O emissions need to be mitigated in the current system.
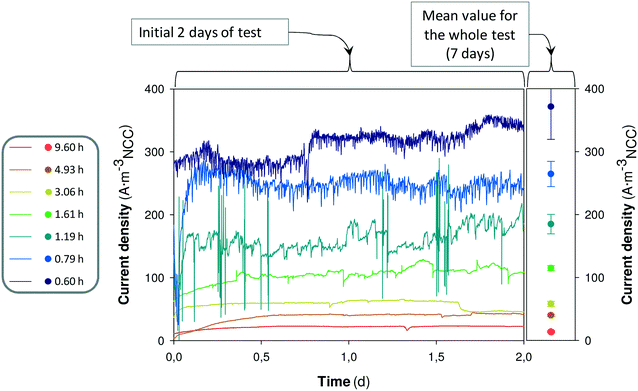
The time denitrifying BES react to the change in the HRT could give an idea about the effect of the HRT on the microbial performance. Fig. 4 shows the on-line response of replicate 1 when changing from one HRT to the next and the mean value of the current density for the whole test (7 days).
The on-line monitoring of current density indicated that, in general, the system had a fast response to the increase of influent flow. In all tests except for the test at 0.60 h, the system already reached a current density similar to the mean value observed for the whole test (7 days), in less than 0.25 days. In this denitrifying BES, the microorganisms responsible for current density and nitrate reduction should be mainly autotrophic, because the influent medium did not contain organic matter. It only contained bicarbonate (inorganic carbon) as a carbon source. Autotrophic microorganisms are known to have slow growth, with estimated maximum growth rates of about 1.0 d−1.33 Hence, the response observed in the denitrifying BES (less than 0.25 days to reach the mean current density observed in the whole test) should be mostly attributed to an increase of bacterial activity rather than bacterial growth. Therefore, these results suggest that the HRT parameter would be the main factor responsible for the increase of the overall performance. However, the variation of HRT already implies both a variation in the water flux (hydrodynamics) and a variation in the nitrate loading rate, as the nitrate influent concentration was constant in all tests. Thus, it could remain unclear whether the hydrodynamics or the nitrate availability has more weighting.
In order to further investigate this point, we decided to evaluate the performance of the denitrifying BES at different influent nitrate concentrations and at different HRTs in the following section.
3.2. Influence of nitrate influent concentration on denitrifying activity at different HRTs
The nitrate removal activity was clearly enhanced when the HRT was decreased. However, the enhancement of denitrifying activity could rely on a better flux distribution itself and/or be due to a higher nitrate loading rate. In order to clear up this uncertainty, the nitrate removal performance was evaluated at different influent nitrate concentrations and at different HRTs. The rNO3− performance against the nitrate influent concentration at different HRTs is shown in Fig. 5.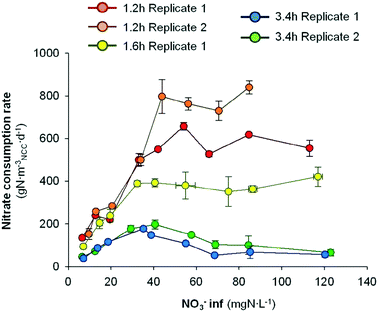 | ||
| Fig. 5 Nitrate consumption rate at different nitrate influent concentrations and at different HRTs. Error bars represent the standard deviation of replicate samples (n = 5). | ||
The evaluation of different nitrate influent concentrations at different HRTs suggested a clear different microbial response as a function of HRT. Again, the overall highest activity was observed at the lowest HRT tested (1.2 h). At this HRT, replicate 1 presented an increase of the rNO3− from 152 ± 25 g N mNCC−3 d−1 to 797 ± 79 g N mNCC−3 d−1 by increasing the nitrate influent concentration from 9.7 to 43.9 mg N L−1. From 43.9 to 70.5 mg N L−1, the nitrate removal activity presented a slight decrease to 730 ± 45 g N mNCC−3 d−1, and then a final increase to 840 ± 31 g N mNCC−3 d−1 at an influent nitrate concentration of 84.7 mg N L−1. Thus, it remained in a range between 730–840 g N mNCC−3 d−1 when the influent nitrate content was between 43.9–84.7 mg N L−1. Replicate 2 at 1.2 h presented a similar trend, but with lower maximum activities.
At an HRT of 1.6 h, the trend was similar to the tests at 1.2 h. An increase of the nitrate removal activity, as the nitrate concentration at the influent increased, of up to 393 ± 18 g N mNCC−3 d−1 was observed when the influent content was 40.6 mg N L−1. At a higher influent NO3− content, the nitrate removal rate slightly decreased until 352 ± 18 g N mNCC−3 d−1 at 75 mg N L−1. Finally, it increased again to 421 ± 45 g N mNCC−3 d−1 at the maximum nitrate influent concentration tested (117.0 mg N L−1). Thus, at influent nitrate concentrations between 40.6–117.0 mg N L−1, the nitrate removal rate was moving in a range between 352–421 g N mNCC−3 d−1.
On the contrary, at the highest HRT (3.4 h), the nitrate removal trend was different. In both replicates, the rNO3− increased to 197 ± 20 and 177 ± 3 g N mNCC−3 d−1 when the nitrate influent concentration was increased to 35.2 and 40.6 mg N L−1 for replicate 1 and 2, respectively. From there up to 113.0 and 120.3 mg N L−1, the nitrate removal rate sharply decreased down to 66 ± 15 and 57 ± 12 g N mNCC−3 d−1 for replicate 1 and 2, respectively. Hence, these results suggested that at higher HRTs, the presence of higher quantities of nitrate in the influent was, somehow, inhibitory for the nitrate reducing bacteria. However, to the author's best knowledge, no nitrate inhibition for denitrifying bacteria has been described yet. A hypothesis for the change of profile for nitrate reduction at high HRTs and high NO3− influent concentrations could be the formation of free nitrous acid because of a higher nitrite accumulation.34 However, no nitrite was observed at the effluent in any test, indicating no nitrite accumulation, thus FNA inhibition could be discarded. Another hypothesis could be the presence of other processes occurring inside the denitrifying BES, in which secondary metabolites could act as inhibitors for denitrifiers. For example, it has been described that plant secondary metabolites like procyanidins are toxic to the denitrifying biomass.35,36 However, this hypothesis could not be evaluated.
At lower HRTs (1.2 h and 1.6 h), the nitrate reducing activity increased with the increase of substrate availability until reaching a flat plateau. By operating the system at 1.2 h, activity stabilization was reached between 43.9 and 54.1 mg N L−1 nitrate influent concentration. By operating the denitrifying BES at 1.6 h, the rNO3− found the stabilization point at lower values (32.3 mg N L−1). And by further increasing the HRT up to 3.4 h, the rNO3− trend changed its shape. A maximum activity between 35.2 and 40.6 mg N L−1, but lower activity was found at either lower or higher nitrate influent values.
On the one hand, these results suggest that the operation at lower HRTs increases the nitrate removal rate because of an increase of the water flow-rate itself, and not due to an increase of nitrate availability. On the other hand, it was observed that, at high HRTs (3.4 h) the increase of nitrate influent concentration above 40.6 mg N L−1 depressed the denitrifying activity.
4. Conclusions
A tubular denitrifying BES was developed for high denitrification rates (up to 849 g N mNCC−3 d−1) at low HRTs (0.6 h) with concomitant anodic pre-disinfection. The nitrate consumption rate in the tubular denitrifying bioelectrochemical systems was promoted by operating at low HRTs. Not only was nitrate reduction enhanced, but the nitrite and nitrous oxide consumption rates were also improved. The whole denitrification process was benefited at lower HRTs. It suggests that an appropriate methodology for scaling-up would be implementing compact reactors (low volume) operated at high HRTs to get high nitrate removal rates and connected in series to reduce effluent nitrate content.The tests of different influent nitrate concentrations at different HRTs revealed different denitrifying activities dependent on substrate availability. At low HRTs (1.2 and 1.6 h), the nitrate reducing activity increased with the increase of nitrate influent concentration until reaching a flat plateau when nitrate influent concentrations are higher than 49 and 32 mg N L−1, respectively. On the contrary, at higher HRTs (3.4 h), the nitrate removal activity presented a Gauss-like chart shape, with the maximum performance at around 38 mg N L−1 and revealing inhibition at higher nitrate influent concentrations.
The results presented here suggested that biological nitrate treatment can be achieved in denitrifying BES at higher rates, competitive treatment-time and with smaller devices. However, in order to scale-up the process and to reach complete nitrate removal, the coupling of different denitrifying BES devices operated at low HRTs would be required.
Conflict of interest
There are no conflicts of interest to declare.Acknowledgements
This research was financially supported by the Spanish Government (CTQ2014-53718-R and CTM2015-71982-REDT) and the University of Girona (MPCUdG2016/137). LEQUIA has been recognized as a consolidated research group by the Catalan Government with code 2014-SGR-1168. Narcís Pous was supported by a project grant from the Catalan Government (2012 FI-B 00941). The authors acknowledge the assistance of Dr. Alba Anfruns and Ms. Gemma Rustullet.References
- S. Ghafari, M. Hasan and M. K. Aroua, Bioresour. Technol., 2008, 99(10), 3965–3974 CrossRef CAS PubMed
.
- P. Clauwaert, K. Rabaey, P. Aelterman, L. De Schamphelaire, T. H. Pham, P. Boeckx, N. Boon and W. Verstraete, Environ. Sci. Technol., 2007, 41(9), 3354–3360 CrossRef CAS PubMed
.
- B. Virdis, K. Rabaey, Z. Yuan, R. A. Rozendal and J. Keller, Environ. Sci. Technol., 2009, 43(13), 5144–5149 CrossRef CAS PubMed
.
- S. Tejedor-Sanz, T. Bacchetti De Gregoris, J. J. Salas, L. Pastor and A. Esteve-Núñez, Environ. Sci.: Water Res. Technol., 2016, 2(5), 884–893 CAS
.
- T. Lotti, R. Kleerebezem, C. Lubello and M. C. M. van Loosdrecht, Water Res., 2014, 60, 1–14 CrossRef CAS PubMed
.
- J. Colt, Aquac. Eng., 2006, 34, 143–156 CrossRef
.
- S. Fox, N. Mozes, O. Lahav, N. Mirzoyan and A. Gross, Water, Air, Soil Pollut., 2015, 226(5), 134 CrossRef
.
- S. Puig, M. Coma, J. Desloover, N. Boon, J. Colprim and M. D. Balaguer, Environ. Sci. Technol., 2012, 46(4), 2309–2315 CrossRef CAS PubMed
.
- Y. Zhang and I. Angelidaki, Water Res., 2013, 47(5), 1827–1836 CrossRef CAS PubMed
.
- N. Pous, S. Puig, M. Coma, M. D. Balaguer and J. Colprim, J. Chem. Technol. Biotechnol., 2013, 88(9), 1690–1696 CrossRef CAS
.
- U. Schröder, F. Harnisch and L. T. Angenent, Energy Environ. Sci., 2015, 8(2), 513–519 Search PubMed
.
- K. B. Gregory, D. R. Bond and D. R. Lovley, Environ. Microbiol., 2004, 6(6), 596–604 CrossRef CAS PubMed
.
- N. Pous, C. Koch, J. Colprim, S. Puig and F. Harnisch, Electrochem. Commun., 2014, 49, 93–97 CrossRef CAS
.
- K. P. Gregoire, S. M. Glaven, J. Hervey, B. Lin and L. M. Tender, J. Electrochem. Soc., 2014, 161(13), H3049–H3057 CrossRef
.
- L. Yu, Y. Yuan, S. Chen, L. Zhuang and S. Zhou, Electrochem. Commun., 2015, 60, 126–130 CrossRef CAS
.
- M. Prosnansky, Y. Sakakibara and M. Kuroda, Water Res., 2002, 36(19), 4801–4810 CrossRef CAS PubMed
.
- Y. Sakakibara and T. Nakayama, Water Res., 2001, 35(3), 768–778 CrossRef CAS PubMed
.
- N. Pous, C. Koch, A. Vilà-Rovira, M. D. Balaguer, J. Colprim, J. Mühlenberg, S. Müller, F. Harnisch and S. Puig, RSC Adv., 2015, 5(84), 68326–68333 RSC
.
- N. Pous, S. Puig, M. D. Balaguer and J. Colprim, Chem. Eng. J., 2015, 263, 151–159 CrossRef CAS
.
- E. J. McAdam and S. J. Judd, Desalination, 2008, 231, 52–60 CrossRef CAS
.
- K. M. Twomey, A. S. Stillwell and M. E. Webber, J. Environ. Monit., 2010, 12, 218–224 RSC
.
- S. Bajracharya, M. Sharma, G. Mohanakrishna, X. Dominguez Benneton, D. P. B. T. B. Strik, P. M. Sarma and D. Pant, Renewable Energy, 2016, 98, 153–170 CrossRef CAS
.
- Y. Zhang and I. Angelidaki, Environ. Sci. Technol., 2016, 50(11), 5432–5433 CrossRef CAS PubMed
.
- P. Clauwaert, J. Desloover, C. Shea, R. Nerenberg, N. Boon and W. Verstraete, Biotechnol. Lett., 2009, 31(10), 1537–1543 CrossRef CAS PubMed
.
- S. J. Ergas and F. Reuss, J. Water Supply: Res. Technol.--AQUA, 2001, 50(3), 161–171 CAS
.
- A. Vilà-Rovira, S. Puig, M. D. Balaguer and J. Colprim, RSC Adv., 2015, 5(96), 78994–79000 RSC
.
- T. Tzedakis and Y. Assouan, Chem. Eng. J., 2014, 253, 427–437 CrossRef CAS
.
- K. Rabaey, W. Ossieur, M. Verhaege and W. Verstraete, Water Sci. Technol., 2005, 52(1–2), 515–523 CAS
.
-
APHA, Standard Methods for the Examination of Water and Wastewater, Washington DC, USA, 19th edn, 2005 Search PubMed
.
- J. Greenman and I. A. Ieropoulos, J. Power Sources, 2017, 356, 365–370 CrossRef CAS
.
- Y. Xiao, Y. Zheng, S. Wu, Z.-H. Yang and F. Zhao, Microb. Ecol., 2014, 69(3), 492–499 CrossRef PubMed
.
- B. E. Logan, B. Hamelers, R. Rozendal, U. Schröder, J. Keller, S. Freguia, P. Aelterman, W. Verstraete and K. Rabaey, Environ. Sci. Technol., 2006, 40(17), 5181–5192 CrossRef CAS PubMed
.
- W. Gujer, M. Henze, T. Mino and M. van Loosdrecht, Water Sci. Technol., 1999, 39(1), 183–193 CrossRef CAS
.
- C. Glass, J. Silverstein and J. Oh, Water Environ. Res., 1997, 69(6), 1086–1093 CrossRef CAS
.
- C. Bardon, F. Piola, F. Bellvert, F. E. Z. Haichar, G. Comte, G. Meiffren, T. Pommier, S. Puijalon, N. Tsafack and F. Poly, New Phytol., 2014, 204(3), 620–630 CrossRef CAS PubMed
.
- C. Bardon, F. Poly, F. E. Z. Haichar, X. Le Roux, L. Simon, G. Meiffren, G. Comte, S. Rouifed and F. Piola, Soil Biol. Biochem., 2017, 107, 41–49 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2017 |