Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Removal rates and energy demand of the electrochemical oxidation of ammonia and organic substances in real stored urine
Hanspeter
Zöllig
 a,
Annette
Remmele
a,
Eberhard
Morgenroth
ab and
Kai M.
Udert
*a
a,
Annette
Remmele
a,
Eberhard
Morgenroth
ab and
Kai M.
Udert
*a
aEawag, Swiss Federal Institute of Aquatic Science and Technology, 8600 Dübendorf, Switzerland. E-mail: kai.udert@eawag.ch; Fax: +41 58 765 5808; Tel: +41 58 765 5360
bETH Zürich, Institute of Environmental Engineering, 8093 Zürich, Switzerland
First published on 10th March 2017
Abstract
The separate collection and treatment of urine allows for an environmentally friendly and cost-efficient management of the nutrients contained in urine. The primary goal should be to recover all these nutrients. However, in some cases it will be economically or ecologically more sensitive to recover only the phosphorus, while nitrogen is removed together with organic substances (measured as chemical oxygen demand, COD) and pathogens. In this study, we investigated the use of galvanostatic electrolysis for the removal of nitrogen and COD from real stored urine. Non-active type boron-doped diamond (BDD) and active type thermally decomposed iridium oxide film (TDIROF) anodes were evaluated using batch experiments. On both anodes, ammonia was exclusively removed by indirect oxidation with active chlorine (AC: Cl2, HClO, and ClO−). As a consequence, ammonia was not completely removed, if chlorine was consumed by competing processes. While COD was present, ammonia removal was faster on TDIROF (227 ± 16 gN m−2 d−1 at 20 mA cm−2) than on BDD (43 ± 20 gN m−2 d−1 at 20 mA cm−2). The reason for the slower ammonia removal on BDD was the enhanced reaction of AC with organic molecules. In fact, hydroxyl radicals broke organic molecules down to shorter chain molecules which reacted with most of the AC leaving only little AC for the oxidation of ammonia. This preferential oxidation of organics resulted in very high COD removal rates on BDD (above 420 gCOD m−2 d−1 at 20 mA cm−2 for COD concentrations above 1000 mgCOD L−1). A main drawback of electrolysis with both anodes was the high energy demand (BDD: 55 W h gCOD−1 and 766 W h gN−1 for 90% and 6% removal, respectively. TDIROF: 67 W h gCOD−1 and 77 W h gN−1 for 30% and 40% removal. All at 20 mA cm−2). It can be concluded that BDD and TDIROF anodes could be combined in series for a fast, complete, and more energy efficient electrochemical urine treatment: COD could be removed on BDD before the residual ammonia would be removed on TDIROF.
Water impactThe separate collection and treatment of urine is a promising approach for innovative sanitation systems. For on-site urine treatment electrolysis could be a robust and compact technology. The article shows that electrolysis can simultaneously remove nitrogen and organic substances at high rates from real stored urine. However, this comes with a price: high energy needs and harmful byproducts. |
1. Introduction
Urine is a concentrated source of nutrients and can be collected separately from the other wastewater streams in waterless urinals or urine-diverting toilets. When fresh urine is excreted by humans, most of the nitrogen (85%) is present as urea and the pH is about 6.4 ± 1.1.1 In stench traps, pipes, and storage tanks, bacteria producing the enzyme urease hydrolyze urea quickly (ureolysis) to bicarbonate and ammonia.2 The result is so called ureolyzed or stored urine with a pH around 9. In stored urine, 90% of the nitrogen is present in the form of ammonia (NH4+ and NH3). Urine treatment processes mostly have to treat stored urine since urine is hardly ever collected under sterile conditions.Several processes were proposed for nutrient recovery from stored urine.3 Some processes, such as evaporation4 or reverse osmosis,5 enable an almost complete separation of all nutrients and water whereas others, such as electrodialysis6 or freeze and thaw methods,7 leave considerable concentrations of the nutrients in the treated effluent (>300 mgN L−1, >60 mgP L−1, >800 mgCOD L−1). Very high nitrogen and COD concentrations, actually close to the ones in the raw urine, remain in the supernatant of the struvite process.8 These residual compounds need to be eliminated from the supernatant of a urine treatment and pathogens must be inactivated.
Electrolysis could be a suitable technology for these purposes especially if compact reactors are desirable for on-site treatment.9 Organic substances as well as urea were successfully removed from synthetic fresh urine for environmental protection.10,11 Other studies used fresh urine as a source of urea for hydrogen production.12,13 It was also shown that electrolysis kills pathogens in fresh urine due to the produced AC (Cl2, HClO, ClO−).14 Additional advantages are that the technology is stand alone, requires low maintenance, and does not rely on the addition of chemicals.9 Furthermore, electrolysis is easy to automate and the treatment progress can be monitored online because the current gives direct information of the reaction rates.15
Only few publications evaluate electrolysis for the removal of ammonia and COD from stored urine. Zheng et al. reported high ammonia oxidation rates on graphite anodes applying high constant currents (50 mA cm−2).16 This very likely was through indirect ammonia oxidation by AC because it was shown recently that direct ammonia oxidation results in much slower removal rates.17 Furthermore, the very high current density must have resulted in the corrosion of the graphite anode18 and the production of chlorination byproducts.19 Zheng et al. reported successful indirect ammonia oxidation on a dimensionally stable anode (DSA, Ti/RuO2–IrO2–TiO2) until specific charges of 3.6 A h L−1.20 However, Amstutz et al. observed the complete inhibition of indirect ammonia oxidation on a similar DSA (Ti/IrO2) when higher specific charges were applied (10 to 80 A h L−1) due to competing carbonate oxidation.21 In the latter two studies, no COD removal was reported but it is known from many other studies in different kinds of wastewater that AC plays an important role for COD removal if chloride is present.22 It remains unclear if complete, simultaneous ammonia and COD removal by electrolytic oxidation is feasible in stored urine and what would be the best operating conditions.
In this work, we discuss three important aspects of simultaneous electrochemical removal of COD and ammonia from real stored urine. In galvanostatic electrolysis experiments in batch reactors we assessed the removal rates of COD and ammonia, determined the necessary specific energy demand, and evaluated the composition of the treated urine. Furthermore, the impacts of three important operating parameters were evaluated: the electrode material (BDD and TDIROF), the current density, and the composition of the raw urine by working with low and high-concentration urine from two different sources. Thus, the presented work adds important knowledge to judge the practical applicability of electrolysis to simultaneously remove nitrogen and organic substances from stored urine.
2. Materials and methods
2.1. Urine electrolysis
The details to the setup of the electrochemical cell were described in a previous publication.19 The most important settings are given here for convenience.An undivided glass cell (400 mL) was equipped either with a boron-doped diamond (Si/BDD, Adamant Technologies SA, La Chaux-de-Fonds, Switzerland) or a thermally decomposed iridium oxide film (TDIROF)23 anode with 20 cm2 of exposed surface area. The cathode with an equivalent surface area was made of steel (X5CrNi18-10, Hans Kohler AG, Zürich, Switzerland). The distance between the electrodes was between 9 and 10 mm. A mercury/mercurous sulfate (MSE) reference electrode was employed to measure the anode potential (EA in V) and was placed in a glass-blown Luggin capillary filled with saturated K2SO4. Temperature and pH were measured continuously (SenTix 41 connected to pH 196, WTW, Weilheim, Germany) and recorded with a data logger. Conductivity was measured with a handheld meter (TetraCon 325 connected to Cond 340i, WTW, Weilheim, Germany).
The electrolysis cell was filled with 350 mL of stored urine which was unequally diluted with intruding flushing water during collection. Only slightly diluted men's urine (because of urinals) and more strongly diluted women's urine were used from the collection tanks at Eawag (Table 1). Electrolysis was performed with a potentiostat (PGU 10V-1A-IMP-S, Ingenieurbüro Peter Schrems, Münster, Germany) which registered EA under galvanostatic control at j = 10, 15 and 20 mA cm−2. A magnetic stirrer ensured turbulence in the reactor and the temperature was controlled with a thermostat (K3 DS, Colora Messtechnik GmbH, Lorch, Germany) at a fixed temperature indicated for each experiment.
| Low-concentration urine | High-concentration urine | ||||
|---|---|---|---|---|---|
| Average | Std. dev. | Average | Std. dev. | ||
| a One measurement. b Five measurements. | |||||
| Total COD | (mg L−1) | 1710 | 50 | 4510 | 290 |
| Total ammonia N | (mg L−1) | 1860 | 40 | 2790 | 170 |
| Nitrite N | (mg L−1) | <0.15 | — | <10 | — |
| Nitrate N | (mg L−1) | <1 | — | <10 | — |
| Chloride | (mg L−1) | 1250 | 130 | 3800 | 150 |
| Total phosphate P | (mg L−1) | 88 | 9 | 242 | 8 |
| Sulfate | (mg L−1) | 234 | 25 | 822 | 45 |
| Total inorganic carbon | (mg L−1) | 903a | — | — | — |
| Conductivity | (mS cm−1) | 15.1a | — | 30.1 | 1.1 |
| pH | (—) | 9.1b | 0.1 | 9 | 0.1 |
| COD/ammonia-N | (mg mg−1) | 0.92 | 0.04 | 1.62 | 0.20 |
| COD/Cl− | (mg mg−1) | 1.38 | 0.13 | 1.18 | 0.05 |
| Ammonia-N/Cl− | (mg mg−1) | 1.50 | 0.15 | 0.74 | 0.05 |
2.2. Chemical analysis
Aliquots (∼13 mL) were taken with a syringe through a needle permanently installed in the glass lid of the reactor. After the total COD was analyzed, the samples were filtered with glass-fiber filters (0.45 μm, Chromafil GF/PET, Macherey-Nagel, Düren, Germany). Chloride, phosphate, sulfate, nitrite, and nitrate were analyzed by ion chromatography (881 compact IC pro, Metrohm, Herisau, Switzerland). Ammonia and total COD were measured photometrically with cuvette tests (LCK 303 and LCK 314/614, Hach Lange, Berlin, Germany). The total inorganic carbon was measured once with a total inorganic/total organic carbon analyzer (TOC-L, Shimadzu, Kyoto, Japan) according to manufacturers' protocol in a sample directly taken from the women's storage tank. The standard deviations of the wet chemical analyses were less than 5%.2.3. Calculations
In this study, strongly mass transfer controlled conditions were only observed for COD degradation on BDD for which the area specific removal rate rCOD (g m−2 d−1) can merely be expressed at a certain COD concentration in the bulk (SCOD,∞ (mg L−1)):24
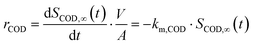 | (1) |
Herein, A (m2) is the electrode surface area, V (m3) is the volume of the electrolyte, t is the time and km,COD (m s−1) is the apparent mass transfer coefficient for COD.
After rearrangement and integration eqn (1) becomes:
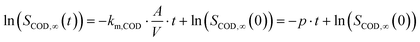 | (2) |
Thus, km,COD was estimated with the initial electrolyte volume Vinit (m3) according to eqn (3):
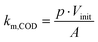 | (3) |
The slope p was taken from the linear regression in the logarithmic plot of the COD concentration against t when COD removal apparently followed first order kinetics.
All other area specific removal or production rates rx (g m−2 d−1) under electron transfer limitation were calculated from a linear regression of the mass (considering the sampling) versus time:
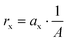 | (4) |
| CENH(t) = CENO3−(t) + CENO2−(t) + CEN2(t) | (5) |
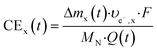 | (6) |
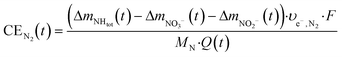 | (7) |
Here, Δmx(t) (gN) is the mass of substance x produced in the cell until time t, and υe−,x (mole− molN−1) is the number of electrons used per mole of ammonia oxidized to nitrate (υe−,NO3− = 8), nitrite (υe−,NO2− = 6) or molecular nitrogen (υe−,N2 = 3), respectively. F is the Faraday constant (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mole−1), MN the molecular weight of nitrogen (14 gN molN−1) and Q(t) (C) the transported charge, calculated from the current I (A) as:
485 C mole−1), MN the molecular weight of nitrogen (14 gN molN−1) and Q(t) (C) the transported charge, calculated from the current I (A) as:
| Q(t) = ∫t0I(t)·dt | (8) |
The current efficiency for organic substance removal (CECOD) was estimated based on COD measurements:
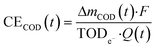 | (9) |
The cumulative specific energy demand for the removal of total ammonia (x = NH) or COD (x = COD) Esp,x (W h g−1) was calculated from the average electrical power ![[U with combining overline]](https://www.rsc.org/images/entities/i_char_0055_0305.gif) ·
·![[I with combining overline]](https://www.rsc.org/images/entities/i_char_0049_0305.gif) , which was calculated as the average of the product of the cell voltage U (V) times the current I (A) up to a time t:
, which was calculated as the average of the product of the cell voltage U (V) times the current I (A) up to a time t:
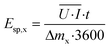 | (10) |
3. Results
3.1. Electrolysis of high-concentration urine
| Urine type | Electrode | j (mA cm−2) | Removal of organic substances | Chloride removal | Ammonia removal | Nitrate production | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r COD | SD | k m,COD | SD | r Cl− | SD | r NH | SD | r NO3-N | SD | |||
| (gCOD m−2 d−1) | (m s−1) | (gCl− m−2 d−1) | (gN m−2 d−1) | (gN m−2 d−1) | ||||||||
| High-concentration urine | BDD | 20 | 421 | 38 | 4.9 × 10−6 | 4.4 × 10−7 | 94 | 14 | 43 | 20 | 51 | 9 |
| 15 | 334 | 26 | 3.9 × 10−6 | 3.0 × 10−7 | 58 | 9 | 87 | 5 | 26 | 1 | ||
| 10 | 190 | 4 | 2.2 × 10−6 | 4.9 × 10−8 | 59 | 6 | 71 | 6 | 28 | 2 | ||
| TDIROF | 20 | 214 | 24 | 174 | 13 | 227 | 16 | 17 | 1 | |||
| 15 | 180 | 24 | 84 | 10 | 184 | 7 | 17 | 1 | ||||
| 10 | 150 | 15 | 20 | 22 | 147 | 5 | 28 | 2 | ||||
| Low-concentration urine | BDD | 20 | 693 | 38 | 8.0 × 10−6 | 4.4 × 10−7 | 268 | 9 | — | — | 95 | 2 |
| 15 | 549 | 28 | 6.4 × 10−6 | 3.2 × 10−7 | 167 | 7 | — | — | 75 | 3 | ||
| 10 | 467 | 26 | 5.4 × 10−6 | 3.0 × 10−7 | 65 | 5 | — | — | 40 | 2 | ||
| TDIROF | 20 | 163 | 13 | 164 | 6 | 257 | 22 | 64 | 7 | |||
| 15 | 133 | 2 | 185 | 22 | 183 | 27 | 55 | 6 | ||||
| 10 | 91 | 6 | 89 | 5 | 110 | 11 | 27 | 1 | ||||
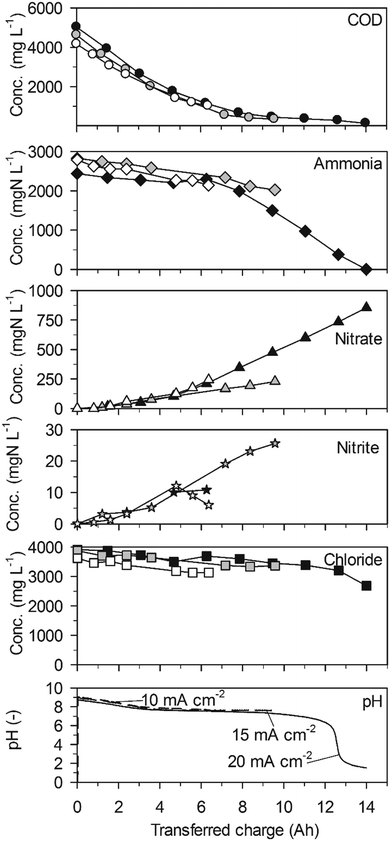
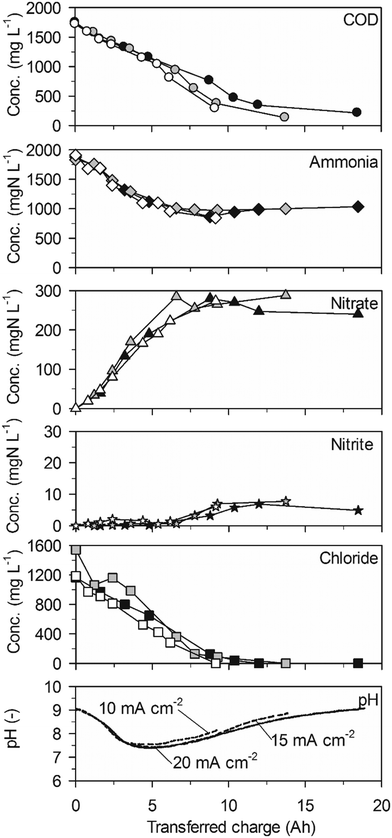
Ammonia was eliminated at much lower rates than COD in a first phase of the experiments up to a transferred charge of 6 A h (Table 2, 43 ± 20 gN m−2 d−1 at 20 mA cm−2, Fig. 1). The preferential oxidation of COD on BDD is also reflected by the high CECOD (Fig. 3). At 20 mA cm−2 ammonia removal accelerated to a much higher rate (419 ± 17 gN m−2 d−1) when the COD was exhausted after a transferred charge of 6 A h. This resulted in complete ammonia removal up to a transferred charge of 14 A h. Simultaneously, chloride was removed at the same constant rate as in the first phase of the experiment (Fig. 1, Table 2). This corroborates that indirect oxidation of organic substances and ammonia were competing reactions on BDD with a preference for COD oxidation.
The two phases of ammonia oxidation led to two different nitrate formation rates in the corresponding time periods at 20 mA cm−2. The nitrate formation rate was 51 ± 9 gN m−2 d−1 in the first phase until 6 A h. This rate increased to 105 ± 2 gN m−2 d−1 in the second phase. The final nitrate yields at current densities of 20, 15 and 10 mA cm−2 were 54.0 ± 37.5%, 26.7 ± 7.4% and 21.8 ± 13.12%, respectively. Simultaneously, only little nitrite accumulated (Fig. 1).
The pH value strongly decreased in the beginning of the experiments until a charge of about 4 A h was transferred (Fig. 1). From then on, the pH value remained at a level of 7.5 due to phosphate buffering (Table 1). At 20 mA cm−2, the buffer capacity of phosphate was exhausted after 12 A h of transferred charge which led to a pH drop to values below 2. This means that the consumption of alkalinity by anodic processes was stronger than alkalinity production by cathodic processes during the whole experiment.
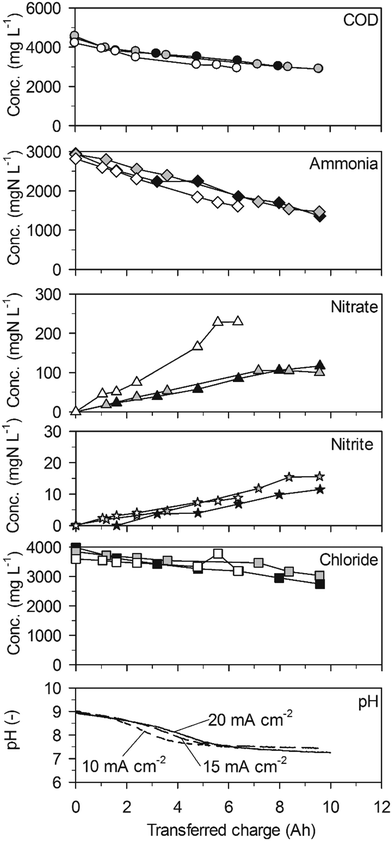
Also ammonia was removed at constant rates (Table 2, 227 ± 16 gN m−2 d−1 maximum at 20 mA cm−2) in parallel to the removal of COD (Fig. 2). The ammonia removal rates increased with the current density and were at least a factor two higher than on the BDD anode. This is in line with the higher chloride removal rates with increasing current density and also with the mostly higher chloride removal rates on TDIROF compared to BDD. At 20 mA cm−2, the chloride removal rate on TDIROF (174 ± 13 g m−2 d−1) was almost double the chloride removal rate observed on BDD (94 ± 14 g m−2 d−1) indicating the importance of indirect ammonia oxidation by AC.
Interestingly, the highest nitrate production rate of 28 ± 2 gN m−2 d−1 was observed at the lowest current density of 10 mA cm−2 (Table 2) and resulted in the highest nitrate yield of 18.0 ± 2.3% which is comparable to the yields observed in low-concentration urine (section 3.2.2). At 20 and 15 mA cm−2, the nitrate yields were comparatively low with 7.1 ± 1.2% and 9.5 ± 2.5%, respectively (Fig. 2). The reason for this could be stronger reduction of nitrate at higher current densities. Nitrite was only formed in small amounts leading to rather low concentrations (Fig. 2).
As with the BDD anode, the pH value dropped in the beginning of the experiments to reach a plateau at pH = 7.5 due to the phosphate buffer system (Fig. 2). About the same charge, 6 A h, was transferred to reach this level with current densities of 15 and 20 mA cm−2 whereas at 10 mA cm−2 only about 4 A h were needed. This corroborates the hypothesis that nitrate reduction was less prevalent at 10 mA cm−2: as we will discuss in section 4.1, cathodic nitrate reduction to nitrite and ammonia sets free hydroxyl ions and thereby buffers some of the protons released at the anode which slows down the decrease of pH.
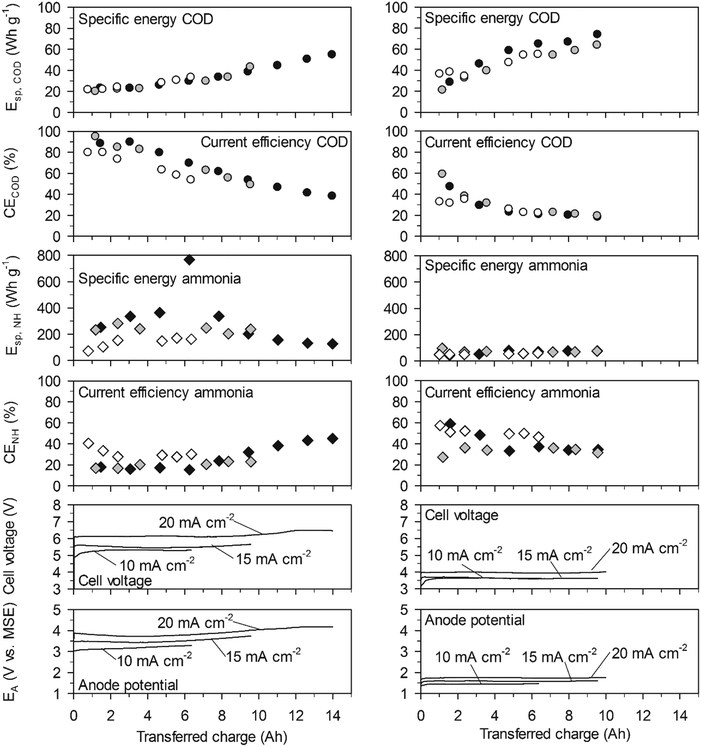
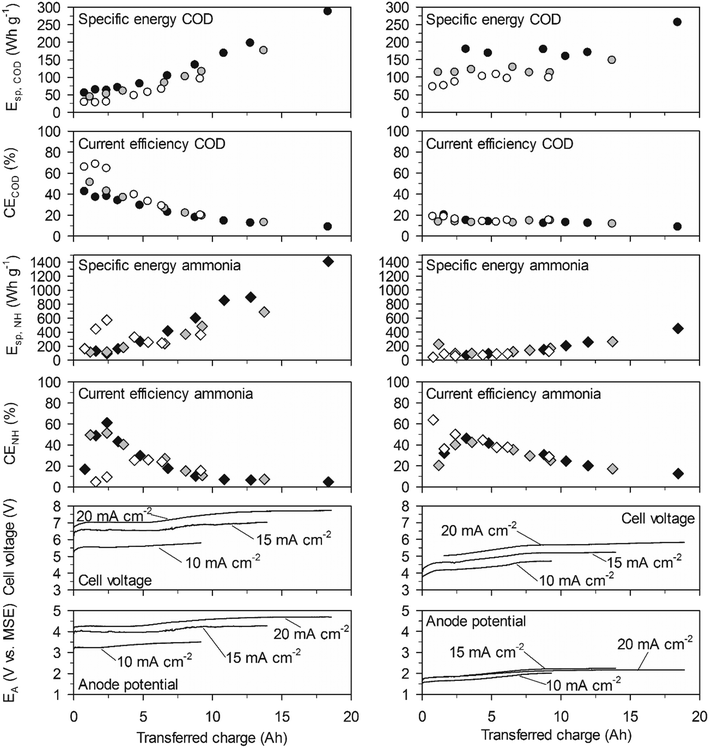
On BDD at 20 mA cm−2, the initially high CECOD of more than 80% resulted in initial values for Esp,COD as low as 23 W h gCOD−1 and for a COD elimination of 90% the Esp,COD was only 55 W h gCOD−1. On TDIROF at 20 mA cm−2, the initial CECOD was below 60% and dropped quickly to values around 30%. As a consequence, the Esp,COD was higher than on BDD and increased from 28 to 67 W h gCOD−1 to achieve a COD elimination of 30%. The higher Esp,COD on TDIROF can be attributed solely to the low CECOD, as the cell voltage was clearly lower than with BDD. These results underline that COD removal is more efficient on BDD.
In contrast, the cumulative specific energy demand for ammonia removal, Esp,NH, was considerably lower on the TDIROF anode. At a current density of 20 mA cm−2Esp,NH increased from 42 to 77 W h gN−1 for the removal of about 40% of the ammonia. On BDD, the Esp,NH was much higher and culminated in a maximum value of 766 W h gN−1 at 20 mA cm−2, after 6.25 A h were transferred and only 6% of the ammonia was removed. The reason for these high Esp,NH values on BDD was the low CENH. This observation underlines the better degradability of ammonia on TDIROF.
3.2. Electrolysis of low-concentration urine
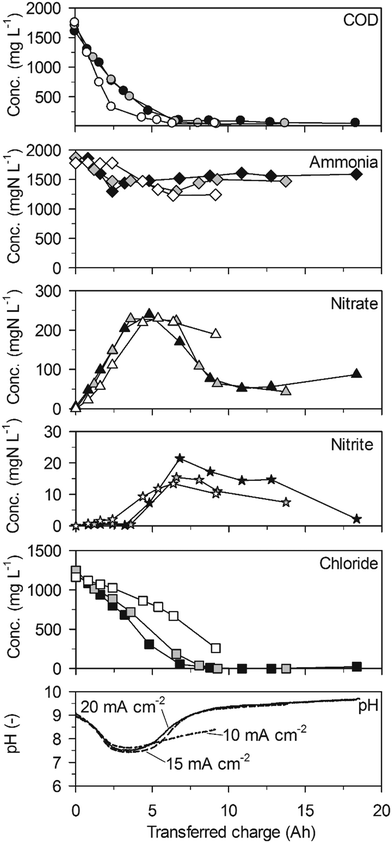
Ammonia was not removed when no chloride was present anymore towards the end of the experiments (Fig. 4, 15 and 20 mA cm−2). However, the constant nitrate formation (Table 2, 95 ± 2 gN m−2 d−1 at 20 mA cm−2) in the beginning of the experiments indicated ammonia oxidation to nitrate when chloride was present. Thus, it can be concluded that ammonia was only oxidized by AC. Unfortunately, it was not possible to calculate ammonia removal rates because the few ammonia data points in the useful range at the beginning of the experiments did not always show a clear trend.
The decreasing nitrate concentrations between 5 and 9 A h clearly indicated that nitrate was not only produced from ammonia but was also cathodically reduced. Nitrate reduction to ammonia can explain the increase of ammonia after 5 A h. The concentrations of nitrite were in a similar range to those in high-concentration urine but showed an intermediate peak at about 7 A h.
Again, the pH value declined from 9 to 7.5 within the first 2.5 A h of transferred charge. This was faster than in high-concentration urine and correlated with a stronger net nitrate production (Table 2). After 5 A h, when net nitrate reduction to ammonia set in, the pH value increased strongly at current densities of 15 and 20 mA cm−2. At 10 mA cm−2, however, the pH value increased more slowly in accordance with a slower net nitrate removal. This effect proves the importance of the interplay of anodic oxidation and cathodic reduction processes of nitrogen species for the course of pH.
Ammonia was oxidized steadily to nitrate at comparable rates as in high-concentration urine (Table 2, 257 ± 22 gN m−2 d−1 maximum at 20 mA cm−2) as long as chloride concentrations were higher than approximately 500 mg L−1 (Fig. 5). However, ammonia removal slowed down considerably when chloride concentrations dropped to lower values. After a transition phase, ammonia removal stopped completely when no chloride was present anymore. Thus, a lack of chloride resulted in incomplete ammonia removal on BDD and TDIROF (Fig. 5).
The phases of net nitrate production (Table 2, 64 ± 7 gN m−2 d−1 maximum at 20 mA cm−2) were in accordance with the periods of ammonia oxidation (Fig. 5). Thereby, the higher production rates than in high-concentration urine led to higher nitrate yields of 25.9 ± 3.1%, 34.0 ± 6.4% and 19.8 ± 5.5% at 20, 15 and 10 mA cm−2, respectively. Similar to the experiments on BDD in low-concentration urine, a slight nitrate removal and a simultaneous increase of the ammonia concentration were observable at 20 mA cm−2 when no chloride was present anymore. This, again, indicates nitrate reduction. Nitrite only appeared when chloride concentrations dropped below approximately 500 mg L−1 and did not exceed 8 mgN L−1.
Similar to the previous experiments, a pH drop was observed during dominant ammonia oxidation to nitrate (Fig. 5). The same minimum pH value of 7.5 was reached but after more transferred charge than with the BDD anode in low-concentration urine. This was in accordance with the slower nitrate production rates on TDIROF (Table 2). In the following, the slowly increasing pH value may be attributed to a slower reduction of nitrate to ammonia compared to the observation on the BDD anode.
On TDIROF at 20 mA cm−2, the Esp,COD was stable at a high level (∼170 W h gCOD−1) up to an elimination of 80% of the COD after 12 A h were transferred. This was a consequence of the near constant and low CECOD (∼15%, Fig. 6). These results confirmed the finding from the experiments in high-concentration urine that COD was degraded more efficiently on BDD due to the higher CECOD.
The Esp,NH was clearly smaller on TDIROF (Fig. 6). The Esp,NH was 90 W h gN−1 on TDIROF at a current density of 20 mA cm−2 when the linear removal came to an end (5 A h). On the BDD anode, Esp,NH was 270 W h gN−1 after the same transferred charge. The better Esp,NH on TDIROF resulted from lower cell voltages and from the higher CENH which confirmed this finding from the experiments in high-concentration urine.
4. Discussion
4.1. Influence of the urine composition
The COD could be eliminated to low levels in all experiments as COD removal was faster with AC but could proceed also without AC. These AC independent processes were the oxidation of organic matter with hydroxyl radicals on BDD and the direct oxidation at the anode surface on TDIROF. Nitrogen, however, could not be eliminated completely and the final composition depended strongly on the initial composition of the urine. When the COD to chloride and, above all, the ammonia to chloride ratios were too high as in low-concentration urine (Table 1) ammonia was only partly oxidized.In such cases, the residual nitrogen species can be explained with a previously established reaction system.26
| 2NH4+ + 3ClO− → N2 + 3H2O + 3Cl− + 2H+ | (11) |
| NH4+ + 4ClO− → H2O + NO3− + 4Cl− + 2H+ | (12) |
| NO2− + HClO → NO3− + Cl− + H+ | (13) |
| NO3− + H2O + 2e− ↔ NO2− + 2OH− | (14) |
| NO2− + 5H2O + 6e− → NH3 + 7OH− | (15) |
| 2NO2− + 4H2O + 6e− → N2 + 8OH− | (16) |
In addition to the oxidation of ammonia to N2 (eqn (11)) and nitrate (eqn (12)), the produced AC oxidized nitrite to nitrate (eqn (13)). Simultaneously, nitrate was electrochemically reduced at the cathode (eqn (14)–(16)).27 In presence of AC in the bulk, nitrite was immediately re-oxidized to nitrate resulting in nitrate accumulation as long as chloride was present. The AC concentration in the bulk got low when chloride oxidation ceased resulting in nitrite appearing in the bulk due to the still strong nitrate reduction at high nitrate concentrations. Simultaneously, nitrite was reduced more strongly to ammonia (eqn (15)) and N2 (eqn (16)), accelerated by higher nitrite concentrations in the cathodic diffusion layer. After a while, an equilibrium was established between nitrite oxidation to nitrate at the anode (eqn (14)) and nitrate reduction to nitrite at the cathode (eqn (14)) leading to a quasi-steady state. However, some nitrite was still reduced according to eqn (15) and very little according to eqn (16). In accordance with these processes, the pH value dropped during dominating ammonia oxidation to N2 and nitrate (eqn (11) and (12)) and increased when nitrite reduction to ammonia or N2 dominated (eqn (15) and (16)).
Complete ammonia removal was achieved with BDD at 20 mA cm−2 in high-concentration urine. However, complete ammonia removal should also be feasible on TDIROF in high-concentration urine. In fact, chloride would have been the last substance being used up based on an extrapolation of the removal rates for COD, ammonia and chloride (Fig. 2). The nitrogen remaining in the urine should than be mainly in the form of nitrate (between 7 and 50% may be expected).
4.2. Removal of COD and ammonia
Ammonia was only removed in the presence of chloride, which was clearly demonstrated in the experiments in low-concentration urine (Fig. 4 and 5). It can be concluded that indirect ammonia oxidation via AC was the responsible process. Neither direct oxidation at the electrode surface nor the oxidation by hydroxyl radicals happened to a great extent. The inhibition of direct ammonia oxidation might be explained with a pH drop in the Nernstian diffusion layer as was found for the TDIROF anode.23 This mechanism is reasonable for BDD as well since Kapałka et al. reported a high pH sensitivity for direct ammonia oxidation on BDD.31 Nevertheless, ammonia was completely removed with BDD although only from high-concentration urine with a low ammonia to chloride ratio (0.74 mgN mgCl−1, Table 1). In low-concentration urine, the ammonia to chloride ratio was higher (1.5 mgN mgCl−1, Table 1) such that not sufficient AC could be produced to remove all ammonia.
In high concentration urine at 20 mA cm−2, two phases with near constant ammonia removal rates could be distinguished. This could be due to the following mechanism. In the first phase, when COD concentrations were high, AC was mainly reacting with organic substances as explained above. Together with the lower AC production rates, this resulted in low indirect ammonia oxidation rates (Table 2). In the second phase, ammonia oxidation increased because more AC was available to react with ammonia as the COD concentrations were already at much lower levels.
As on BDD, AC was inevitable for substantial oxidation of ammonia on the TDIROF anode (Fig. 5). Interestingly, carbonate oxidation did not outcompete chloride oxidation in real urine in contrast to the findings in synthetic urine by Amstutz et al.21 The reason for this could be the lower pH values and the lower total carbonate content in real stored urine (Table 1) leading to less CO32− which is the reactant for carbonate oxidation. Direct ammonia oxidation at the anode surface was probably largely inhibited by a pH drop in the Nernstian diffusion layer and could not contribute to ammonia removal.23
Nevertheless, the ammonia removal rates were clearly higher on TDIROF (Table 2) than on BDD and up to two orders of magnitude higher than in biofilm systems (2.5 gN m−2 d−1).33 This suggested a better availability of AC for the oxidation of ammonia on the TDIROF anode. The reason for this was that AC was not used up in reactions with short chain organic substances as on BDD. On TDIROF, the reactions of AC with longer chain organic substances were slower19 and resulted in more available AC for ammonia oxidation also at high COD concentrations. However, also with the TDIROF anode the ammonia to chloride ratio was too high in low-concentration urine (1.5 mgN mgCl−1, Table 1) to achieve complete ammonia removal.
4.3. Specific energy demand
On TDIROF, Esp,COD was generally higher than on BDD but the dependence on the COD concentration was identical. COD removal on TDIROF required between 1.7 and 3 times more energy than COD removal on BDD even though the cell voltages were lower. The cause was the much lower CECOD on TDIROF. This contradicts findings in synthetic urine by Dbira et al. who found a lower Esp,COD (105 W h gCOD−1, 95% elimination) with an IrO2–RuO2 anode which might be caused by a different CECOD in their experiments.11
Compared to other COD reduction technologies the energy demand of electrolysis was very high. In the partial nitrification of urine, the elimination of COD was observed as a side effect. Maurer et al. estimated an energy demand of 54 MJ m−3 for the removal of 82% COD, when the initial COD concentration was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mgCOD L−1.3 This corresponds to an Esp,COD value of 1.83 W h gCOD−1. Other technologies cannot completely eliminate but reduce COD in the main urine stream. Electrodialysis could reduce COD by 90% requiring 30 kW h m−3 of energy.3 The resulting Esp,COD value is 3.00 W h gCOD−1. Also evaporation has a lower energy demand to separate COD. A 99% removal of COD from the distillate required 400 MJ m−3 which corresponds to an Esp,COD value of 11.1 W h gCOD−1.3
000 mgCOD L−1.3 This corresponds to an Esp,COD value of 1.83 W h gCOD−1. Other technologies cannot completely eliminate but reduce COD in the main urine stream. Electrodialysis could reduce COD by 90% requiring 30 kW h m−3 of energy.3 The resulting Esp,COD value is 3.00 W h gCOD−1. Also evaporation has a lower energy demand to separate COD. A 99% removal of COD from the distillate required 400 MJ m−3 which corresponds to an Esp,COD value of 11.1 W h gCOD−1.3
The energy demand for ammonia oxidation on TDIROF was in a similar range compared to other studies using electrolysis for ammonia removal in synthetic urine. Zheng et al. reported an energy demand for indirect ammonia oxidation of 73 W h gN−1 at an applied current density of 50 mA cm−2 with another type of DSA anode (RuO2–IrO2–TiO2/Ti).20 The same group estimated the energy demand for indirect ammonia oxidation with graphite anodes to 103 W h gN−1. Electrochemically, a lower Esp,NH of 42 W h gN−1 was only achieved with direct ammonia oxidation on a graphite anode.17
Nitrogen could also be removed from source-separated urine with other technologies such as nitrification/denitrification with an organic electron donor or with the anammox process.35 However, urine contains too little organic substances for complete nitrification/denitrification.36 Only the anammox process was investigated in more detail but no experimental energy needs were reported.37,38 Maurer et al. estimated the required electrical energy for the anammox process with urine to 1.7 W h gN−1 which is considerably less than what was required for electrolysis.3
4.4. Applying electrolysis for urine treatment
Our experiments show that COD and ammonia can be removed from real stored urine with galvanostatic electrolysis. However, the simultaneous removal of ammonia and COD is only possible on TDIROF and in the presence of chloride. Nevertheless, the high removal rates indeed make electrolysis interesting for compact urine treatment reactors.For a complete removal of organic substances and ammonia a serial combination of electrolysis cells could be interesting. In the first cell with a BDD anode, organic substances would be removed preferentially via the two oxidation pathways as described above. This would make more chloride available for the subsequent ammonia oxidation in the second cell that would preferably be equipped with a TDIROF anode to decrease the energy demand. In such a system it could also be possible to treat urine with unfavorable COD to chloride and ammonia to chloride ratios.
Despite these promising aspects of electrochemical COD and ammonia removal it must be noted that harmful byproducts were formed in oxidation processes with AC. In a recent publication, we have shown that the removed chloride was mainly oxidized to chlorate and perchlorate on both anodes depending on the duration of the treatment.19 After the complete elimination of COD and ammonia, it has to be expected that perchlorate is the dominant chlorine species. This poses a severe environmental risk.39 Furthermore, the study of Zöllig et al. showed that organic chlorination byproducts were formed which were mainly stripped into the gas phase.19
5. Conclusions
• On both anodes, ammonia can only be removed substantially by indirect oxidation with AC. However, COD removal also consumes AC, especially on TDIROF and to a smaller extent on BDD. Therefore, the ratios of COD to chloride and ammonia to chloride should be low to enable complete COD and ammonia removal. This means that stored urine with low ammonia concentrations, e.g., due to ammonia volatilization, is more suitable for electrochemical nitrogen removal. Also, chloride dosage can enhance electrochemical ammonia removal.• BDD is more efficient for the elimination of COD while TDIROF is better suited for the elimination of ammonia at high COD concentrations (above 1000 mgCOD L−1). The efficient COD elimination on BDD results from two oxidation pathways that aid one another. The oxidation via hydroxyl radicals produces smaller organic molecules which react efficiently with AC leaving little AC for ammonia oxidation. On TDIROF, the produced AC reacts with organics but also with ammonia.
• The difference in the reaction mechanisms on BDD and TDIROF anodes could be exploited by combining both electrodes in a serial reactor setup. The preferential oxidation of COD on BDD in a first cell should result in more available active chlorine for indirect ammonia oxidation in the subsequent cell with a TDIROF anode. In this way, complete COD and ammonia removal may be achieved even in urine with high ratios of COD to chloride and ammonia to chloride.
• Besides the formation of chlorination byproducts the high specific energy demand remains a main drawback of electrochemical COD and ammonia removal. It results from low current efficiencies and high cell voltages. The current efficiencies may be increased by decreasing the current density continuously with decreasing reactant concentrations. The cell voltage can be reduced by minimizing ohmic losses and by reducing the overpotentials at the anode and the cathode.
Acknowledgements
We thank Karin Rottermann and Claudia Bänninger-Werffeli for the chemical analysis. Furthermore, we thank the Bill and Melinda Gates Foundation (OPP1011603) for financing this study as part of the VUNA project (www.vuna.ch).References
- E. Friedler, D. Butler and Y. Alfiya, in Source Separation and Decentralization for Wastewater Management, ed. T. A. Larsen, K. M. Udert and J. Lienert, IWA Publishing, London, UK, 2013, ch. 17, pp. 241–257 Search PubMed.
- K. M. Udert, T. A. Larsen, M. Biebow and W. Gujer, Water Res., 2003, 37, 2571–2582 CrossRef CAS PubMed.
- M. Maurer, W. Pronk and T. A. Larsen, Water Res., 2006, 40, 3151–3166 CrossRef CAS PubMed.
- K. M. Udert and M. Wächter, Water Res., 2012, 46, 453–464 CrossRef CAS PubMed.
- M. Ek, R. Bergström, J. E. Bjurhem, B. Björlenius and D. Hellström, Water Sci. Technol., 2006, 54, 437–444 CrossRef CAS PubMed.
- W. Pronk, M. Biebow and M. Boller, Environ. Sci. Technol., 2006, 40, 2414–2420 CrossRef CAS PubMed.
- B. B. Lind, Z. Ban and S. Bydén, Ecol. Eng., 2001, 16, 561–566 CrossRef.
- B. Etter, E. Tilley, R. Khadka and K. M. Udert, Water Res., 2011, 45, 852–862 CrossRef CAS PubMed.
- K. M. Udert, S. Brown-Malker and J. Keller, in Source Separation and Decentralization for Wastewater Management, ed. T. A. Larsen, K. M. Udert and J. Lienert, IWA Publishing, London, UK, 2013, ch. 22, pp. 321–335 Search PubMed.
- S. Dbira, N. Bensalah, A. Bedoui, P. Canizares and M. A. Rodrigo, Environ. Sci. Pollut. Res. Int., 2015, 22, 6176–6184 CrossRef CAS PubMed.
- S. Dbira, N. Bensalah, P. Canizares, M. A. Rodrigo and A. Bedoui, J. Electroanal. Chem., 2015, 744, 62–68 CrossRef CAS.
- J. Kim, W. J. K. Choi, J. Choi, M. R. Hoffmann and H. Park, Catal. Today, 2013, 199, 2–7 CrossRef CAS.
- B. K. Boggs, R. L. King and G. G. Botte, Chem. Commun., 2009, 4859–4861, 10.1039/b905974a.
- A. S. Raut, G. B. Cunningham, C. B. Parker, E. J. D. Klem, B. R. Stoner, M. A. Deshusses and J. T. Glass, J. Electrochem. Soc., 2014, 161, G81–G85 CrossRef.
- A. Anglada, A. Urtiaga and I. Ortiz, J. Chem. Technol. Biotechnol., 2009, 84, 1747–1755 CrossRef CAS.
- X. Y. Zheng, X. B. Wu, H. R. Ye, L. Yan, Y. J. Zhang, T. X. Cheng, C. Wang and H. N. Kong, The removal of ammonia from source separated urine by electrolytic oxidization using graphite electrodes, Proceedings of 2009 Beijing International Environmental Technology Conference, Water & Wastewater Engineering Magazine Publ, Beijing, 2009, pp. 300–308 Search PubMed.
- H. Zöllig, C. Fritzsche, E. Morgenroth and K. M. Udert, Water Res., 2015, 69, 284–294 CrossRef PubMed.
- M. Rueffer, D. Bejan and N. J. Bunce, Electrochim. Acta, 2011, 56, 2246–2253 CrossRef CAS.
- H. Zöllig, A. Remmele, C. Fritzsche, E. Morgenroth and K. M. Udert, Environ. Sci. Technol., 2015, 49, 11062–11069 CrossRef PubMed.
- X. Y. Zheng, L. A. Ji, H. R. Ye, Y. J. Zhang, L. Yan, J. Li, C. Wang and H. N. Kong, Fresenius Environ. Bull., 2010, 19, 991–998 CAS.
- V. Amstutz, A. Katsaounis, A. Kapałka, C. Comninellis and K. M. Udert, J. Appl. Electrochem., 2012, 42, 787–795 CrossRef CAS.
- M. Panizza and G. Cerisola, Chem. Rev., 2009, 109, 6541–6569 CrossRef CAS PubMed.
- H. Zöllig, E. Morgenroth and K. M. Udert, Electrochim. Acta, 2015, 165, 348–355 CrossRef.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, John Wiley & Sons, New York, 2001 Search PubMed.
- G. Fóti, D. Gandini, C. Comninellis, A. Perret and W. Haenni, Electrochem. Solid-State Lett., 1999, 2, 228–230 CrossRef.
- G. Perez, R. Ibanez, A. M. Urtiaga and I. Ortiz, Chem. Eng. J., 2012, 197, 475–482 CrossRef CAS.
- E. Lacasa, P. Canizares, J. Llanos and M. A. Rodrigo, J. Hazard. Mater., 2012, 213, 478–484 CrossRef PubMed.
- W. Gujer, Siedlungswasserwirtschaft, Springer, Berlin, 2002 Search PubMed.
- J. Boudreau, D. Bejan and N. J. Bunce, Can. J. Chem., 2010, 88, 418–425 CrossRef CAS.
- M. Panizza and G. Cerisola, Electrochim. Acta, 2004, 49, 3221–3226 CrossRef CAS.
- A. Kapałka, L. Joss, A. Anglada, C. Comninellis and K. M. Udert, Electrochem. Commun., 2010, 12, 1714–1717 CrossRef.
- A. Y. Bagastyo, D. J. Batstone, K. Rabaey and J. Radjenovic, Water Res., 2013, 47, 242–250 CrossRef CAS PubMed.
- G. Tchobanoglous, F. L. Burton and H. D. Stensel, Wastewater engineering: Treatment and reuse, Metcalf & Eddy, Inc., McGraw-Hill, Boston, 2003 Search PubMed.
- S. Dbira, N. Bensalah, A. Bedoui, P. Canizares and M. A. Rodrigo, Environ. Sci. Pollut. Res., 2014, 22, 6176–6184 CrossRef PubMed.
- H. Bürgmann, S. Jenni, F. Vazquez and K. M. Udert, Appl. Environ. Microbiol., 2011, 77, 5897–5907 CrossRef PubMed.
- K. M. Udert and S. Jenni, in Source Separation and Decentralization for Wastewater Management, ed. T. A. Larsen, K. M. Udert and J. Lienert, IWA Publishing, London, UK, 2013, ch. 20, pp. 291–306 Search PubMed.
- K. M. Udert, E. Kind, M. Teunissen, S. Jenni and T. A. Larsen, Water Sci. Technol., 2008, 58, 277–284 CrossRef CAS PubMed.
- K. M. Udert, C. Fux, M. Munster, T. A. Larsen, H. Siegrist and W. Gujer, Water Sci. Technol., 2003, 48, 119–130 CAS.
- E. T. Urbansky, Environ. Sci. Pollut. Res., 2002, 9, 187–192 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |