Research highlights: investigating the role of nanoparticle surface charge in nano–bio interactions
Caley
Allen
*a,
Tian A.
Qiu
b,
Sunipa
Pramanik
b,
Joseph T.
Buchman
b,
Miriam O. P.
Krause
c and
Catherine J.
Murphy
d
aDepartment of Chemistry, Johns Hopkins University, Baltimore, MD 21218, USA. E-mail: callen71@jhu.edu
bDepartment of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA
cCenter for Sustainable Nanotechnology, Department of Chemistry, University of Minnesota, Minneapolis, MN 55455, USA
dDepartment of Chemistry, University of Illinois at Urbana-Champaign, Urbana, IL 61801, USA
First published on 4th April 2017
Abstract
A systematic approach to predicting nanoparticle–cell interactions has become increasingly important due to the great potential that nanoparticles hold for biomedical and environmental applications. However, the quantitative description and accurate characterization of nanomaterial surface chemistry (e.g., ligand distribution and surface charge) is nontrivial due to the sheer complexity of both the nanoparticle mechanisms and the biological environments with which they interact. The authors of this highlight, including both experimental and theoretical chemists, were motivated to explore the current gap in the fundamental knowledge about nanoparticle surface charge-dependent interactions across a variety of biological systems. The highlight focuses on three recent publications that survey the effects of nanoparticle surface charge across several bio-system complexities, addressing: (i) ligand-coated gold nanoparticles traversing a lipid bilayer, (ii) silica nanoparticle uptake into human osteoblast cells, and (iii) the suborgan distribution of gold nanoparticles in mice.
Introduction
Many applications of nanomaterials hinge on the critical interactions that occur at the nano–bio interface. Researchers often focus on studying the shape, size, and surface composition of the nanomaterials to investigate the microenvironment at the cell surface. However, the mechanisms of nanomaterials crossing the cell membrane to reach the interior of the cell often involve additional factors, making this process difficult to understand, let alone predict. One physicochemical nanoparticle (NP) property that plays a key role in lipid membrane interactions is the surface charge of the nanomaterial, which can be moderately controlled by attaching certain ligands to the particle's surface. Correlating specific surface charge of nanomaterials with respect to their behavior at the nano–bio interface can offer insights into the corresponding biological signaling, particle transportation, cellular toxicity, and environmental impact.A variety of methods are used to characterize nanomaterial surface charge and ligand distribution, a complex task because the nanomaterials are in diverse environments with a plethora of potential interactions that can modify their surface chemistry. For example, NPs are often functionalized with long hydrocarbon chains that possess titratable functional groups, such as amines, sulfonates, alcohols, and carboxylate groups that face the solvent. These charged nanoparticles will therefore interact with other charged species in the surrounding environment, resulting in the surface modification of the nanoparticles, and the possible formation of complex coronas. Through the continued integration of experimental investigations with computational analysis and modeling techniques, an increased understanding of the relationship between surface charge and its impacts on biological systems will continue to unfold.
Three publications are highlighted here based on their contributions to understanding the complex effect of nanomaterial surface charge across various biologically-relevant systems. The first manuscript details the computational investigation of ligand-coated gold nanoparticles (AuNPs) translocating across symmetric and asymmetric lipid bilayers. The second article describes how the customized surface charge of silica nanoparticles affects uptake into human osteoblast cells; the last investigates how surface charge affects the suborgan distribution of AuNPs in mice. Through the continued systematic investigation of nanoparticle surface charge effects at the nano–bio interface, researchers in the field will continue to make advances toward creating and designing smart, environmentally considerate nanosystems with predictable biological mechanisms.
Molecular simulation to understand nanoparticle–membrane interactions
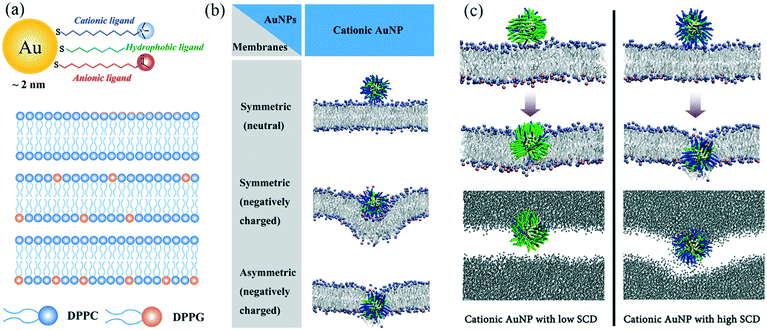
Understanding how nanoparticles translocate through cell membranes is pivotal in designing nanoparticles that can enter cells for biomedical applications without causing toxic effects. In a recent study, Quan et al. (Quan et al., Langmuir, 2017, 33, 361–371, DOI: 10.1021/acs.langmuir.6b02937) used computational simulations to investigate the interactions between different types of lipid bilayers and surface-functionalized AuNPs with different surface charges. This study reveals the roles of lipid membrane composition and nanoparticle surface charge density in penetration of NPs through lipid bilayers.Using the coarse-grained molecular dynamics MARTINI force field, AuNPs of 2 nm diameter were constructed and decorated with three types of ligands on the surface. Each AuNP surface was coated with 100 ligands of varying properties. Negatively charged, neutral (hydrophobic), and positively charged ligands were represented as three hydrophobic beads plus a head group bead with negative, hydrophobic, and positive charge, respectively (Fig. 1a). Three lipid membranes were constructed using zwitterionic dipalmitoylphosphatidylcholine (DPPC) and negatively-charged dipalmitoylphosphatidylglycerol (DPPG): one symmetric bilayer composed of DPPC only, one symmetric bilayer mainly composed of DPPC with DPPG distributed at both inner and outer leaflets, and one asymmetric bilayer composed of DPPC with DPPG only in the inner leaflet (Fig. 1a).
Results showed that, firstly, the anionic AuNPs bind to all three kinds of membranes, possibly due to the weak electrostatic interactions between the negative charge on the AuNP surface and the positively-charged choline head groups on the lipids. However, there is no driving force for these AuNPs to further penetrate the symmetric or asymmetric bilayers. On the other hand, the hydrophobic AuNPs cannot adsorb onto the lipid membrane because of the lack of favorable electrostatic interactions with the bilayers. Interestingly, membrane symmetry determines how cationic AuNPs interact with the membrane (Fig. 1b). Cationic AuNPs showed an increased affinity to the DPPG-doped membranes. The penetration of cationic AuNPs through the membrane only happens for asymmetric bilayers, indicating the strong attraction between the charged groups on the inner leaflet and the AuNPs. Additional simulations were carried out with increased ionic strength, which clearly illustrated how these favorable electrostatic interactions are important for the AuNP penetration mechanism.
To further investigate the mechanism and driving force of cationic AuNPs penetrating an asymmetric lipid membrane, the authors altered the surface charge density (SCD) on the AuNP surface by changing the ratio of positively charged head groups and hydrophobic head groups. Results show that all cationic AuNPs with different SCDs can penetrate the membrane, but different mechanisms of penetration exist for AuNPs of low SCD and high SCD (Fig. 1c). AuNPs of 10% charge density adsorb and stay on the membrane surface for a relatively long time (100 ns) until one lipid molecule protrudes out of the outer leaflet, leading to a favorable hydrophobic contact between lipid tails and the hydrophobic chains of AuNP ligands. The AuNP finally stabilizes at a thermodynamically preferred “snorkeling” configuration, with the ligand chains deforming to expose charged end-groups to the aqueous interface. During this reorganization, no water molecules enter the membrane, and the membrane is not bent. On the other hand, AuNPs of 70% charge density quickly attach and bend the membrane, and pierce the membrane core at about 12.4 ns. This quick AuNP penetration causes the formation of a hydrophilic hole, which in turn causes water leakage into the membrane. Moreover, some of the negatively charged DPPG ligands undergo a flip-flop mechanism and switch from the inner leaflet to the outer leaflet. The authors conclude that hydrophobic interactions are the driving force for the penetration of AuNPs with low SCD, while electrostatic attraction drives the penetration of AuNPs with high SCD.
These observations from simulation can help us to better understand experimental results. For example, cationic AuNPs are found to be more toxic to cells, generally, than anionic AuNPs. The mechanisms behind toxicity can be explained by the membrane disruption and liquid exchange between the medium and cytosol, which is a result of the hydrophilic holes in the membrane caused by cationic AuNPs. More importantly, as cellular uptake (or membrane penetration) and cytotoxicity are always co-players, computational simulations such as presented in this work can be instructive for experimentalists who aim to design nanoparticles balancing both safety and functionality. However, some of the results presented in this work are based on simulations without the addition of salt and a large effective screening constant was used within the non-polarizable MARTINI force field. Therefore, careful consideration must be made when concluding what impact and role the salt plays in the AuNP membrane penetration mechanism.
Effects of silica NPs with customized surface charge on uptake in human osteoblast cells
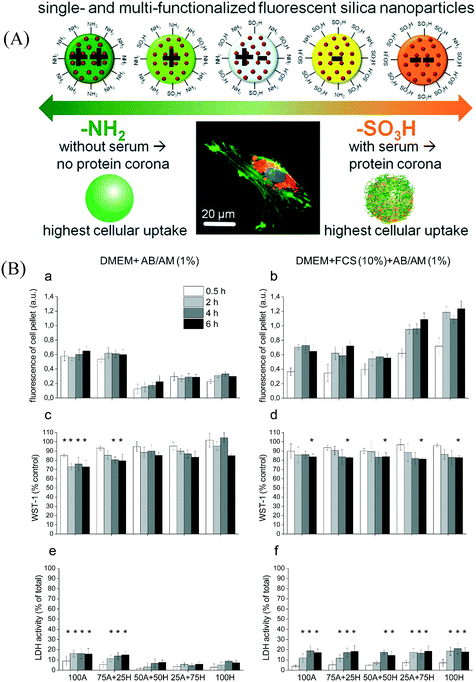
Shahabi et al. (Shahabi et al., ACS Appl. Mater. Interfaces, 2015, 7, 13821–13833, DOI: 10.1021/acsami.5b01900) studied the effect of NP surface charge on NP–cell interactions and NP internalization in the presence and absence of serum proteins, and further investigated the mechanisms of nanoparticle uptake in human osteoblast (HOB) cells. For this purpose, a library of functionalized fluorescent silica nanoparticles (FFSNPs) was synthesized by functionalizing their surfaces with different ratios of amino and sulfonate groups. The FFSNPs had similar physicochemical characteristics, such as size and hydrophilicity, but a controlled gradation of zeta potentials (Fig. 2A). The authors used a water-in-oil reverse microemulsion process to synthesize these nanoparticles, with the fluorescent rhodamine B isothiocyanate (RBITC) dye covalently incorporated to make them fluorescent. To regulate surface charge on the particles, they introduced 3-aminopropyl-triethoxysilane (APTES) and 3-(trihydroxysilyl)-1-propanesulfonic acid (HSPSA) at different ratios (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 75
0, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 50
25, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 25
50, 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 and 0
75 and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100).
100).
The zeta potentials of the FFSNPs in aqueous dispersions were reported to be in a range from 33.4 ± 4.5 to −53.7 ± 3.5 mV, a similar trend to that observed in serum-free media containing Dulbecco's modified eagle medium (DMEM) and 1% antibiotic–antimycotic (AB/AM). All the particles achieved a negative zeta potential in the serum-containing media comprising DMEM, 10% fetal calf serum (FCS), and 1% AB/AM. This indicates that the serum formed a protein corona around the NPs. Particle sizing via dynamic light scattering showed that the FFSNPs were colloidally stable in serum-rich media, possibly due to the protein corona. However, the NPs showed moderate aggregation tendencies in aqueous dispersions and rapid aggregation in serum-free media with high electrolyte concentration due to the high ionic strength of the media.
The internalization of the FFSNPs by the HOB cells was qualitatively visualized using fluorescence microscopy and quantitatively assessed by measuring the fluorescence intensity of the cell pellets after they had been incubated with the particles. The cell samples were also treated with LysoTracker, a blue fluorescent dye that stains lysosomes. Colocalization of FFSNPs and lysosome showed that FFSNPs accumulated in the lysosomes. From the fluorescence microscopy images, authors also reported that all FFSNPs of different surface charges were internalized by the HOB cells, irrespective of the presence or absence of serum proteins in the media. The fluorimetry experiments revealed that in serum-free media, more positively charged FFSNPs than their negatively charged counterparts were incorporated into the cells (Fig. 2B). This is probably due to the electrostatic attraction of the positively charged FFSNPs for the negatively charged domains on cell membranes. However, in serum-rich media, the initially negatively charged NPs accumulated more in the cells. This may be due to the different composition of the protein corona around the positively charged and negatively charged NPs, which could contribute to their propensity towards adsorbing different proteins from the media.
Shahabi et al. assessed the viability of the HOB cells in the presence of the FFSNPs through the water-soluble tetrazolium salt (WST-1) cell proliferation assay and monitored the activities of cytosolic enzyme lactate dehydrogenase (LDH) using the Pierce assay. The WST-1 assay ascertains cell viability by colorimetric quantification of the reduction of tetrazolium salt WST-1 to formazan by cellular mitochondrial dehydrogenases, which is dependent on the glycolytic production of nicotinamide adenine dinucleotide phosphate in healthy cells. In serum-free media, cationic NPs decreased the cleavage of WST-1, indicating decreased cell viability; whereas in the presence of serum, all FFSNPs caused a decrease in cell viability after relatively long exposure (Fig. 2B). LDH is a cytosolic enzyme that can get released through damaged plasma membranes. There was an increase in extracellular LDH detected in the presence of positively charged FFSNPs in serum-free media, and in the presence of all the FFSNPs in serum-rich media. The incorporation of the FFSNPs was correlated with the toxicity effects of the NPs, as obtained from fluorimetry and fluorescence microscopy results.
The uptake of both positively charged and negatively charged NPs in the HOB cells decreased significantly at 4 °C, which can be attributed to a decrease in endocytic pathways. This was further corroborated by experiments in which treatment of cells with an endocytosis inhibitor, such as chlorpromazine, caused a drastic decrease in the uptake of all NPs into the cells, regardless of the presence or absence of serum in media. However, an experiment using the micropinocytosis inhibitor, wortmannin, inhibited NP accumulation only in serum-rich media, indicating that micropinocytosis was a contributing mechanism only in serum-containing media.
From the studies of Shahabi et al., it was concluded that there was an increase in the incorporation of anionic NPs in the cells in serum-rich media, whereas the presence or absence of serum proteins did not affect the intake of positively charged NPs. But as the authors point out, contradictory findings can be found in the literature regarding the uptake of NPs in the presence and absence of serum. This can be attributed to a plethora of reasons such as NP properties, cell structures, and composition of media. Thus, it is difficult to reach a unifying conclusion regarding the correlation of surface charge of NPs and their cellular incorporation, and more research is necessary (for instance, measuring bound molecules to NPs during cellular internalization) in order to understand this complex phenomenon.
The effect of surface charge on gold nanoparticle suborgan distribution in mice
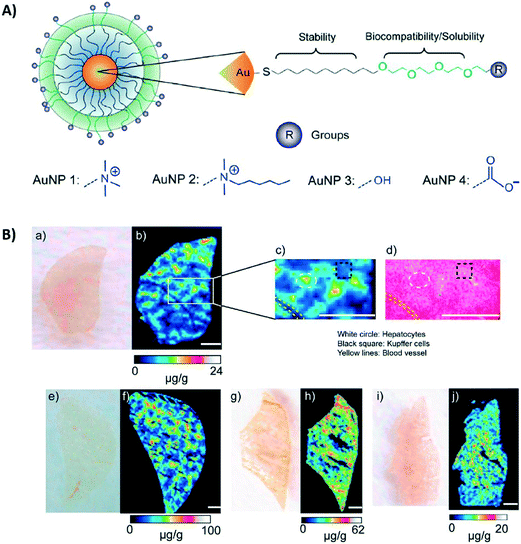
Elci et al. (Elci et al., ACS Nano, 2016, 10, 5536–5542, DOI: 10.1021/acsnano.6b02086) used mice, an even more complex model than the previous two papers, to investigate how surface charge affects the biodistribution of 2-nm AuNPs. The nanoparticles were functionalized with ligands (Fig. 3A), which differed only in their headgroups. The different charges exhibited by each of the AuNPs in this study were due to the identity of this headgroup, which was either a positively charged amine (AuNP 1 and 2), a neutral alcohol group (AuNP 3), or a negatively charged carboxylate group (AuNP 4). The initial zeta potentials in 5 mM phosphate buffer (pH 7.4) for AuNP 1, AuNP 2, AuNP 3, and AuNP 4 were measured to be 21.1 ± 0.5, 16.6 ± 0.6, −0.8 ± 7.6, and −41.2 ± 1.7 mV, respectively.Twenty-four hours after injection into the tail vein of the mice, the AuNP content in the organs was quantified using inductively coupled plasma mass spectrometry (ICP-MS) to check the gold distribution among organs. The spleen and liver contained the highest amount of AuNPs compared to the other organs that were tested, such as the lungs and heart. This is not surprising given that these organs are involved in the clearance of NPs from the body. Both the liver and the spleen had higher levels of positively charged AuNPs than neutral or negatively charged AuNPs.
To investigate the suborgan distribution, the authors used a quantitative imaging technique, laser ablation ICP-MS (LA-ICP-MS). This technique allowed them to capture images that also included quantifiable information about the amount of gold present. Fig. 3B shows examples of these images, specifically the distribution of the AuNPs in the liver. The positively charged AuNPs ended up in the hepatocytes and endothelium, with very little ending up in Kupffer cells, which serve a role in immune response as they work to remove pathogens and waste materials from the body. On the other hand, the neutral and, to a lesser extent, negatively charged AuNPs had more accumulation in Kupffer cells, but were in general more broadly distributed throughout the whole organ.
Looking further at AuNPs in the spleen showed that different initial surface charges led to different distributions within three different areas: red pulp, white pulp, and the marginal zone. Red pulp is tissue that cleans blood by removing particulate matter and dead blood cells; white pulp has a role in the host immune system by producing antibodies and storing white blood cells. The marginal zone is where the immune response is initiated, as this is the area where the matter moves from the red pulp to the white pulp. While all of the AuNPs accumulated in the red pulp, the neutral and negatively charged AuNPs had a larger accumulation in the white pulp and marginal zone. In contrast, the positively charged AuNPs showed very little presence in the white pulp and marginal zone.
Finally, within the kidney, the positively charged AuNPs accumulated in the glomeruli, which is where the filtering process of the kidney is initiated. The neutral AuNPs ended up in the arteries in the kidney and the negatively charged AuNPs were broadly distributed throughout the organ.
These different distributions suggest that the positively charged AuNPs are more quickly filtered by the body than the others, since they accumulate in tissues with roles in filtration. Further evidence that the positively charged AuNPs are more quickly cleared is that they are not seen in the liver blood vessels while neutral and negatively charged AuNPs are. Of the AuNPs tested, the neutral NPs accumulate the most in tissues involved in immune response, which suggests that the neutral NPs are the most likely to interact with the immune system in the host. The authors speculate that this happens because the neutral NPs might acquire a more immune-competent protein corona comprising proteins like IgG or fibrinogen. The negatively charged AuNPs interact less with the immune system than the neutral AuNPs and are not cleared as quickly as the positively charged AuNPs. This knowledge of initial surface charge effects on NP distribution within an organism is useful for the intentional design of NPs suited for specific nanotherapeutic applications.
Acknowledgements
This highlight was initiated from the literature discussion of a biweekly trainee (student and research scientist) group meeting in the Center for Sustainable Nanotechnology, which is supported by the National Science Foundation under grant number CHE-1503408.| This journal is © The Royal Society of Chemistry 2017 |